Chemical data page for ethanol
This page provides supplementary chemical data on ethanol .
Material Safety Data Sheet
External MSDS
structure of ethanol
Structure and properties
Thermodynamic properties
Phase behavior
Triple point
150 K (−123 °C), 0.00043 Pa
Critical point
514 K (241 °C), 63 bar
Std enthalpy change , Δfus H o +4.9 kJ/mol
Std entropy change , Δfus S o +31 J/(mol·K)
Std enthalpy change , Δvap H o ± 0.4[ 4]
Std entropy change , Δvap S o 109.67 J/(mol·K)
Molal freezing point constant
−1.99 °C kg/mol
Solid properties
Std enthalpy change , Δf H o solid
−277.7 kJ/mol
Standard molar entropy ,S o solid
160.7 J/(mol K)[ 5]
Heat capacity , cp
111.46 J/(mol K)[ 5]
Liquid properties
Std enthalpy change , Δf H o liquid
−277.38 kJ/mol
Standard molar entropy ,S o liquid
159.9 J/(mol K)
Enthalpy of combustion , Δc H o −1370.7 kJ/mol
Heat capacity , cp
112.4 J/(mol K)
Gas properties
Std enthalpy change , Δf H o gas
−235.3 kJ/mol
Standard molar entropy ,S o gas
283 J/(mol K)
Heat capacity ,[ 7] cp
78.28 J/(mol K) at 90 °C
Heat capacity ratio ,[ 7] γ = cp /cv
1.13 at 90 °C
van der Waals' constants
a = 1217.9 L2 kPa/mol2 b = 0.08407 L/mol
Spectral data
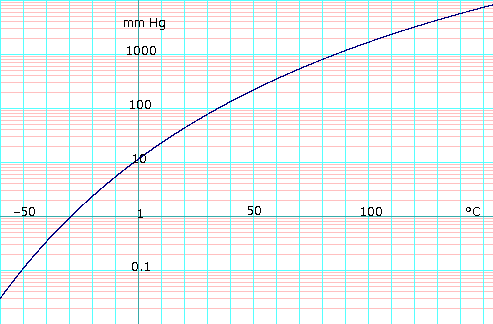
Vapor pressure of liquid
P in mm Hg
1
10
40
100
400
760
1,520
3,800
7,600
15,200
30,400
45,600
P in Pa
133.3
1,333
5,333
13,332
53,329
101,325
202,650
506,625
1,013,250
2,026,500
4,053,000
6,079,501
T in °C
−31.3
−2.3
19.0
34.9
63.5
78.4
97.5
126.0
151.8
183.0
218.0
242.0
T in K
241.85
270.85
292.15
308.05
336.65
351.55
370.65
399.15
424.95
456.15
491.15
515.15
Ethanol vapor pressure vs. temperature. Uses formula
P
mm Hg
=
10
8.04494
−
1554.3
222.65
+
T
{\displaystyle P_{\text{mm Hg}}=10^{8.04494-{\frac {1554.3}{222.65+T}}}}
Log10 of ethanol vapor pressure vs. temperature. Uses formula
log
10
P
mm Hg
=
8.04494
−
1554.3
222.65
+
T
{\displaystyle \log _{10}P_{\text{mm Hg}}=8.04494-{\tfrac {1554.3}{222.65+T}}}
Density of ethanol at various temperatures
Data obtained from Lange 1967
T °Cρ, g/cm3
T °Cρ, g/cm3
T °Cρ, g/cm3
3
0.80374
16
0.79283
29
0.78182
4
0.80290
17
0.79198
30
0.78097
5
0.80207
18
0.79114
31
0.78012
6
0.80123
19
0.79029
32
0.77927
7
0.80039
20
0.78945
33
0.77841
8
0.79956
21
0.78860
34
0.77756
9
0.79872
22
0.78775
35
0.77671
10
0.79788
23
0.78691
36
0.77585
11
0.79704
24
0.78606
37
0.77500
12
0.79620
25
0.78522
38
0.77414
39
0.77329
40
0.77244
These data correlate as ρ [g/cm3 ] = −8.461834× 10T [°C] + 0.8063372 with an R 2
Properties of aqueous ethanol solutions
Data obtained from Lange 1967
Mass fraction ,Volume concentration ,Mass concentration , g/(100 ml) at 15.56 °C
Density relative to 4 °C water [citation needed
Density at 20 °C relative to 20 °C water
Density at 25 °C relative to 25 °C water
Freezing temperature,
10 °C
20 °C
25 °C
30 °C
0.0
0.0
0.0
0.99973
0.99823
0.99708
0.99568
1.00000
1.00000
0
1.0
0.99785
0.99636
0.99520
0.99379
0.99813
0.99811
2.0
0.99602
0.99453
0.99336
0.99194
0.99629
0.99627
2.5
3.13
0.99363
−1
3.0
0.99426
0.99275
0.99157
0.99014
0.99451
0.99447
4.0
5.00
3.97
0.99258
0.99103
0.98984
0.98839
0.99279
0.99274
4.8
6.00
4.76
0.98971
−2
5.0
0.99098
0.98938
0.98817
0.98670
0.99113
0.99106
5.05
6.30
5.00
0.98930
6.0
0.98946
0.98780
0.98656
0.98507
0.98955
0.98945
6.8
8.47
0.98658
−3
7.0
0.98801
0.98627
0.98500
0.98347
0.98802
0.98788
8.0
0.98660
0.98478
0.98346
0.98189
0.98653
0.98634
9.0
0.98524
0.98331
0.98193
0.98031
0.98505
0.98481
10.0
12.40
9.84
0.98393
0.98187
0.98043
0.97875
0.98361
0.98330
11.0
0.98267
0.98047
0.97897
0.97723
0.98221
0.98184
11.3
14.0
11.11
0.98006
−5
12.0
15.0
0.98145
0.97910
0.97753
0.97573
0.98084
0.98039
13.0
0.98026
0.97775
0.97611
0.97424
0.97948
0.97897
13.78
17.00
13.49
−6.1
14.0
0.97911
0.97643
0.97472
0.97278
0.97816
0.97757
15.0
0.97800
0.97514
0.97334
0.97133
0.97687
0.97619
15.02
18.50
14.68
0.97511
16.0
0.97692
0.97387
0.97199
0.96990
0.97560
0.97484
16.4
20.2
0.97336
−7.5
17.0
0.97583
0.97259
0.97062
0.96844
0.97431
0.97346
17.5
21.5
0.97194
−8.7
18.0
22.10
17.54
0.97473
0.97129
0.96923
0.96697
0.97301
0.97207
18.8
23.1
0.97024
−9.4
19.0
0.97363
0.96997
0.96782
0.96547
0.97169
0.97065
20.0
0.97252
0.96864
0.96639
0.96395
0.97036
0.96922
20.01
24.50
19.44
0.96863
20.3
24.8
0.96823
−10.6
21.0
0.97139
0.96729
0.96495
0.96242
0.96901
0.96778
22.0
0.97024
0.96592
0.96348
0.96087
0.96763
0.96630
22.11
27.00
21.43
0.96578
−12.2
23.0
0.96907
0.96453
0.96199
0.95929
0.96624
0.96481
24.0
0.96787
0.96312
0.96048
0.95769
0.96483
0.96329
24.2
29.5
0.96283
−14.0
25.0
30.40
24.12
0.96665
0.96168
0.95895
0.95607
0.96339
0.96176
26.0
0.96539
0.96020
0.95738
0.95422
0.96190
0.96018
26.7
32.4
0.95914
−16.0
27.0
0.96406
0.95867
0.95576
0.95272
0.96037
0.95856
28.0
33.90
26.90
0.96268
0.95710
0.95410
0.95098
0.95880
0.95689
29.0
0.96125
0.95548
0.95241
0.94922
0.95717
0.95520
29.9
36.1
0.95400
−18.9
30.0
36.20
28.73
0.95977
0.95382
0.95067
0.94741
0.95551
0.95345
31.0
0.95823
0.95212
0.94890
0.94557
0.95381
0.95168
32.0
0.95665
0.95038
0.94709
0.94370
0.95207
0.94986
33.0
0.95502
0.94860
0.94525
0.94180
0.95028
0.94802
33.8
40.5
0.94715
−23.6
34.0
0.95334
0.94679
0.94337
0.93986
0.94847
0.94613
35.0
0.95162
0.94494
0.94146
0.93790
0.94662
0.94422
35.04
41.90
33.25
0.94486
36.0
0.94986
0.94306
0.93952
0.93591
0.94473
0.94227
37.0
0.94805
0.94114
0.93756
0.93390
0.94281
0.94031
38.0
0.94620
0.93919
0.93556
0.93186
0.94086
0.93830
39.0
46.3
0.94431
0.93720
0.93353
0.92979
0.93886
0.93626
−28.7
40.0
0.94238
0.93518
0.93148
0.92770
0.93684
0.93421
40.04
47.40
37.61
0.93510
41.0
0.94042
0.93314
0.92940
0.92558
0.93479
0.93212
42.0
0.93842
0.93107
0.92729
0.92344
0.93272
0.93001
43.0
0.93639
0.92897
0.92516
0.92128
0.93062
0.92787
44.0
0.93433
0.92685
0.92301
0.91910
0.92849
0.92571
45.0
0.93226
0.92472
0.92085
0.91692
0.92636
0.92355
45.31
53.00
42.07
0.92406
46.0
0.93017
0.92257
0.91868
0.91472
0.92421
0.92137
46.3
53.8
0.92193
−33.9
47.0
0.92806
0.92041
0.91649
0.91250
0.92204
0.91917
48.0
0.92593
0.91823
0.91429
0.91028
0.91986
0.91697
49.0
0.92379
0.91604
0.91208
0.90805
0.91766
0.91475
50.0
0.92162
0.91384
0.90985
0.90580
0.91546
0.91251
50.16
58.0
46.04
0.91349
51.0
0.91943
0.91160
0.90760
0.90353
0.91322
0.91026
52.0
0.91723
0.90936
0.90524
0.90125
0.91097
0.90799
53.0
0.91502
0.90711
0.90307
0.89896
0.90872
0.90571
54.0
0.91279
0.90485
0.90079
0.89667
0.90645
0.90343
55.0
0.91055
0.90258
0.89850
0.89437
0.90418
0.90113
55.16
63.0
50.00
0.90220
56.0
0.90831
0.90031
0.89621
0.89206
0.90191
0.89833
56.1
63.6
0.90008
−41.0
57.0
0.90607
0.89803
0.89392
0.88975
0.89962
0.89654
58.0
0.90381
0.89574
0.89162
0.88744
0.89733
0.89423
59.0
0.90154
0.89344
0.88931
0.88512
0.89502
0.89191
60.0
0.89927
0.89113
0.88699
0.88278
0.89271
0.88959
60.33
68.0
53.98
0.89038
61.0
0.89698
0.88882
0.88466
0.88044
0.89040
0.88725
62.0
0.89468
0.88650
0.88233
0.87809
0.88807
0.88491
63.0
0.89237
0.88417
0.87998
0.87574
0.88574
0.88256
64.0
0.89006
0.88183
0.87763
0.87337
0.88339
0.88020
65.0
0.88774
0.87948
0.87527
0.87100
0.88104
0.87783
66.0
0.88541
0.87713
0.87291
0.86863
0.87869
0.87547
67.0
0.88308
0.87477
0.87054
0.86625
0.87632
0.87309
68.0
0.88071
0.87241
0.86817
0.86387
0.87396
0.87071
69.0
0.87839
0.87004
0.86579
0.86148
0.87158
0.86833
70.0
0.87602
0.86766
0.86340
0.85908
0.86920
0.86593
71.0
0.87365
0.86527
0.86100
0.85667
0.86680
0.86352
71.9
78.3
0.86311
−51.3
72.0
0.87127
0.86287
0.85859
0.85426
0.86440
0.86110
73.0
0.86888
0.86047
0.85618
0.85184
0.86200
0.85869
74.0
0.86648
0.85806
0.85376
0.84941
0.85958
0.85626
75.0
0.86408
0.85564
0.85135
0.84698
0.85716
0.85383
76.0
0.86168
0.85322
0.84891
0.84455
0.85473
0.85140
77.0
0.85927
0.85079
0.84647
0.84211
0.85230
0.84895
78.0
0.85685
0.84835
0.84403
0.83966
0.84985
0.84650
79.0
0.85422
0.84590
0.84158
0.83720
0.84740
0.84404
80.0
0.85197
0.84344
0.83911
0.83473
0.84494
0.84157
81.0
0.84950
0.84096
0.83664
0.83224
0.84245
0.83909
82.0
0.84702
0.83848
0.83415
0.82974
0.83997
0.83659
83.0
0.84453
0.83599
0.83164
0.82724
0.83747
0.83408
84.0
0.84203
0.83348
0.82913
0.82473
0.83496
0.83156
85.0
0.83951
0.83095
0.82660
0.82220
0.83242
0.82902
86.0
0.83697
0.82840
0.82405
0.81965
0.82987
0.82646
87.0
0.83441
0.82583
0.82148
0.81708
0.82729
0.82389
88.0
0.83181
0.82323
0.81888
0.81448
0.82469
0.82128
89.0
0.82919
0.82062
0.81626
0.81186
0.82207
0.81865
90.0
0.82654
0.81797
0.81362
0.80922
0.81942
0.81600
91.00
94.00
74.62
0.82386
0.81529
0.81094
0.80655
0.81674
0.81331
92.0
0.82114
0.81257
0.80823
0.80384
0.81401
0.81060
93.0
0.81839
0.80983
0.80549
0.80111
0.81127
0.80785
94.0
0.81561
0.80705
0.80272
0.79835
0.80848
0.80507
95.0
0.81278
0.80424
0.79991
0.79555
0.80567
0.80225
96.0
0.80991
0.80138
0.79706
0.79271
0.80280
0.79939
97.0
0.80698
0.79846
0.79415
0.78981
0.79988
0.79648
98.0
0.80399
0.79547
0.79117
0.78684
0.79688
0.79349
99.0
0.80094
0.79243
0.78814
0.78382
0.79383
0.79045
100.0
100.0
79.39
0.79784
0.78934
0.78506
0.78075
0.79074
0.78736
−114.3
Mass fraction,
Volume concentration,
Mass concentration, g/(100 ml) at 15.56 °C
Density relative to 4 °C water
Density at 20 °C relative to 20 °C water
Density at 25 °C relative to 25 °C water
Freezing temperature,
10 °C
20 °C
25 °C
30 °C
Boiling points of aqueous solutions
Data obtained from CRC Handbook of Chemistry (Page 2117) [ 7] : 2391
BP °C
Weight % ethanol
BP °C
Weight % ethanol
liquid vapor liquid vapor
78.1
95.5‡
95.5‡
78.2
91
92
86.5
18
71
78.4
85
89
87.0
17
70
78.6
82
88
87.5
16
69
78.8
80
87
88.0
15
68
79.0
78
86
88.5
13
67
79.2
76
85
89.0
12
65
79.4
74
85
89.5
11
63
79.6
72
84
90.0
10
61
79.8
69
84
90.5
10
59
80.0
67
83
91.0
9
57
80.2
64
83
91.5
8
55
80.4
62
82
92.0
8
53
80.6
59
82
92.5
7
51
80.8
56
81
93.0
6
49
81.0
53
81
93.5
6
46
81.2
50
80
94.0
5
44
81.4
47
80
94.5
5
42
81.6
45
80
95.0
4
39
81.8
43
79
95.5
4
36
82.0
41
79
96.0
3
33
82.5
36
78
96.5
3
30
83.0
33
78
97.0
2
27
83.5
30
77
97.5
2
23
84.0
27
77
98.0
1
19
84.5
25
75
98.5
1
15
85.0
23
74
99.0
< 1
10
85.5
21
73
99.5
< 1
5
86.0
20
72
100.0
0
0
‡ Azeotropic mixture
Charts
Thermophysical properties of mixtures of ethanol with water and dodecane
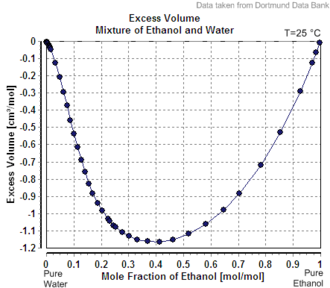
Excess volume of the mixture of ethanol and water (volume contraction)
Heat of mixing of the mixture of ethanol and water
Vapor–liquid equilibrium of the mixture of ethanol and water (including azeotrope )
Solid–liquid equilibrium of the mixture of ethanol and water (including eutecticum )
Miscibility gap in the mixture of dodecane and ethanol
References
^ NMR-002: Sample Devices and Magnetic Susceptibility ^ Touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical properties of matter – the TPRC data series. Volume 3. Thermal conductivity – nonmetallic liquids and gases. Data book. 1970.
^ "Pure Component Properties" (Queriable database) . Chemical Engineering Research Information Center. Retrieved 12 May 2007 .^ "Ethanol" . webbook.nist.gov . Retrieved 7 December 2021 .^ a b Atkins, Peter; de Paula, Julio (2010). Atkins' Physical Chemistry 913– 947. ISBN 978-0-19-954337-3 ^ a b c Hodgman, Charles D; Weast, Robert C; Shankland, Robert S; Selby, Samuel M (1963). CRC Handbook of chemistry and physics : a ready-reference book of chemical and physical data (44th ed.). Cleveland Ohio: The Chemical Rubber Publishing. pp. 2582– 2584. ^ "Spectral Database for Organic Compounds" (Queriable database) . Advanced Industrial Science and Technology. Retrieved 9 June 2007 .
Lange, Norbert Adolph (1967). John Aurie Dean (ed.). Lange's Handbook of Chemistry Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook , NIST Standard Reference Database Number 69