Chemical data page
This page provides supplementary chemical data on o -Xylene
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS ) for this chemical from a reliable source such as eChemPortal , and follow its directions. MSDS is available from MATHESON TRI-GAS, INC. in the SDSdata.org database.
Structure and properties
Thermodynamic properties
Phase behavior
Triple point
247.8 K (−25.3 °C), ? Pa
Critical point
631 K (358 °C), 3700 kPa
Std enthalpy change , Δfus H o 13.6 kJ/mol
Std entropy change , Δfus S o 54.87 J/(mol·K) at −25.3
Std enthalpy change , Δvap H o 36.24 kJ/mol at 144.5°C
Std entropy change , Δvap S o ? J/(mol·K)
Solid properties
Std enthalpy change , Δf H o solid
? kJ/mol
Standard molar entropy ,S o solid
? J/(mol K)
Heat capacity , cp
? J/(mol K)
Liquid properties
Std enthalpy change , Δf H o liquid
−24.4 kJ/mol
Standard molar entropy ,S o liquid
247 J/(mol K)
Enthalpy of combustion , Δc H o −4552 kJ/mol
Heat capacity , cp
187.0 J/(mol K) at 25°C
Gas properties
Std enthalpy change , Δf H o gas
19.0 kJ/mol
Standard molar entropy ,S o gas
353.6 J/(mol K)
Heat capacity , cp
132.5 J/(mol K) at 25°C
van der Waals' constants [ 6] a = 3038 L2 kPa/mol2
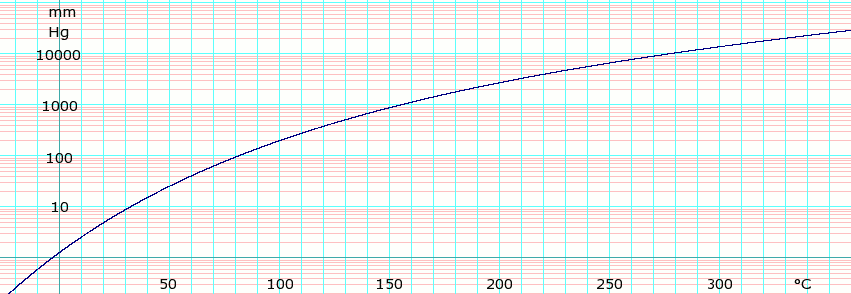
Vapor pressure of liquid
P in mm Hg 1
10
40
100
400
760
T in °C −3.8
32.1
59.5
81.3
121.7
144.4
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
log10 of o -Xylene vapor pressure. Uses formula:
log
e
P
m
m
H
g
=
{\displaystyle \scriptstyle \log _{e}P_{mmHg}=}
log
e
(
760
101.325
)
−
10.06059
log
e
(
T
+
273.15
)
−
7946.229
T
+
273.15
+
83.32184
+
5.939742
×
10
−
6
(
T
+
273.15
)
2
{\displaystyle \scriptstyle \log _{e}({\frac {760}{101.325}})-10.06059\log _{e}(T+273.15)-{\frac {7946.229}{T+273.15}}+83.32184+5.939742\times 10^{-6}(T+273.15)^{2}}
[ 7]
Distillation data
See also:
Vapor-liquid Equilibriumo -Xylene/m -Xylene [ 8] P = 26.66 kPa
BP
% by mole m -xylene
liquid
vapor
100.1
0.0
0.0
99.8
4.7
5.6
99.6
9.7
11.4
99.2
17.8
20.6
98.8
25.9
29.3
98.4
34.1
38.1
97.9
42.5
46.8
97.6
49.6
53.9
97.2
57.4
61.5
96.8
65.5
69.2
96.5
73.5
76.5
96.1
80.8
83.2
95.8
88.6
90.2
95.6
93.7
94.6
95.3
100.0
100.0
Vapor-liquid Equilibriumo -Xylene/Carbon tetrachloride [ 8] P = 760 mm Hg
BP
% by mole carbon tetrachloride
liquid
vapor
142.0
1.8
6.5
136.0
6.4
22.9
130.4
11.2
36.9
125.0
16.1
49.4
120.7
20.4
57.3
116.1
25.3
64.6
111.8
30.2
70.9
107.9
35.2
75.9
104.2
40.4
80.3
104.1
40.5
80.5
99.4
47.8
85.2
95.1
55.4
88.9
91.6
62.4
91.6
88.2
70.0
94.0
85.3
77.1
95.8
82.6
84.1
97.2
80.0
91.0
98.5
77.7
97.6
99.6
Vapor-liquid Equilibriumo -Xylene/Ethanol [ 8] P = 101.3 kPa
BP
% by mole ethanol
liquid
vapor
78.3
99.52
99.66
78.7
98.59
99.03
78.7
97.73
98.52
79.0
95.78
97.40
79.3
90.59
95.09
79.7
86.79
94.16
80.0
85.01
93.70
80.2
80.91
92.91
80.3
76.77
92.36
82.0
67.69
91.21
83.8
48.66
89.25
88.1
25.11
87.32
97.3
10.94
81.49
112.8
5.22
65.91
137.3
0.65
22.90
Vapor-liquid Equilibriumo -Xylene/Methanol [ 8] P = 101.3 kPa
BP
% by mole Methanol
liquid
vapor
64.7
98.78
99.10
64.8
98.33
98.81
64.8
97.67
98.45
64.9
96.70
97.96
65.3
93.34
96.82
65.7
86.84
95.68
66.3
84.24
95.31
66.7
83.30
95.65
67.3
76.74
94.97
68.4
49.33
94.52
71.5
21.76
93.84
102.1
2.49
80.00
Spectral data
References
^ Merck Index of Chemicals and Drugs , 9th ed. monograph 9743^ CRC Handbook of Chemistry and Physics , 44th ed. pp 2611–2620^ CRC Handbook of Chemistry and Physics , 44th ed. pp 2244–2248^ Lange's Handbook of Chemistry , 10th ed. pp 1669–1674^ CRC Handbook of Chemistry and Physics , 85th ed. p8-111^ Lange's Handbook of Chemistry , 10th ed, pp 1522–1524^ "Pure Component Properties" (Queriable Database) . Chemical Engineering Research Information Center. Retrieved 27 May 2007 .^ a b c d "Binary Vapor-Liquid Equilibrium Data" (Queriable database) . Chemical Engineering Research Information Center. Retrieved 27 May 2007 .