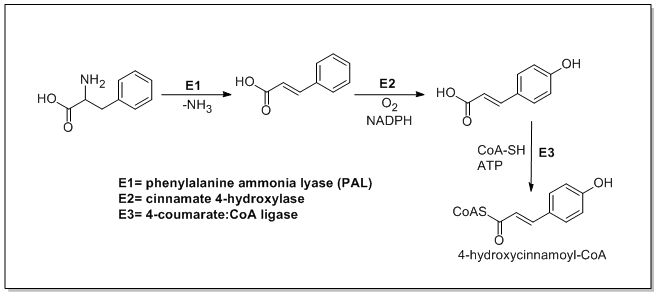
Phenylpropanoids metabolismThe biosynthesis of phenylpropanoids involves a number of enzymes. From amino acids to cinnamatesIn plants, all phenylpropanoids are derived from the amino acids phenylalanine and tyrosine. Phenylalanine ammonia-lyase (PAL, a.k.a. phenylalanine/tyrosine ammonia-lyase) is an enzyme that transforms L-phenylalanine and tyrosine into trans-cinnamic acid and p-coumaric acid, respectively. Trans-cinnamate 4-monooxygenase (cinnamate 4-hydroxylase) is the enzyme that transforms trans-cinnamate into 4-hydroxycinnamate (p-coumaric acid). 4-Coumarate-CoA ligase is the enzyme that transforms 4-coumarate (p-coumaric acid) into 4-coumaroyl-CoA.[1] Enzymes associated with biosynthesis of hydroxycinnamic acids
Conjugation enzymesThese enzymes conjugate phenylpropanoids to other molecules.
Deconjugation enzymes
Stilbenoids biosynthesis
An alternative bacterial ketosynthase-directed stilbenoids biosynthesis pathway exists in Photorhabdus bacterial symbionts of Heterorhabditis nematodes, producing 3,5-dihydroxy-4-isopropyl-trans-stilbene for antibiotic purposes.[2] Coumarins biosynthesis
Chalcones biosynthesis4-Coumaroyl-CoA can be combined with malonyl-CoA to yield the true backbone of flavonoids, a group of compounds called chalconoids, which contain two phenyl rings. Naringenin-chalcone synthase is an enzyme that catalyzes the following conversion:
Flavonoids biosynthesisConjugate ring-closure of chalcones results in the familiar form of flavonoids, the three-ringed structure of a flavone. BiodegradationHydroxycinnamic acids degradation
References
|
Portal di Ensiklopedia Dunia