µŁżµóØńø«ķ£ĆĶ”ü
ń▓ŠķĆܵł¢ń夵éēńøĖÕģ│õĖ╗ķóśńÜäń╝¢ĶĆģ ÕÅéõĖÄÕÅŖÕŹÅÕŖ®ń╝¢ĶŠæŃĆé
(2017Õ╣┤12µ£ł16µŚź ) Ķ½ŗķéĆĶ½ŗ ķü®ÕÉłńÜäõ║║ÕŻ½µö╣բ䵣¼µØĪńø« ŃĆéµø┤ÕżÜńÜäń┤░ń»ĆĶłćĶ®│µāģĶ½ŗÕÅāĶ¦üĶ©ÄĶ½¢ķĀü ŃĆé
ń╗┤Õ¤║ńÖŠń¦æ õĖŁńÜäķå½ÕŁĖÕåģÕ«╣
õ╗ģõŠøÕÅéĶĆā ’╝īõĖ”
õĖŹĶāĮ Ķ”¢õĮ£Õ░łµźŁµäÅĶ”ŗŃĆéÕ”éķ£ĆńŹ▓ÕÅ¢ķå½ńÖéÕ╣½ÕŖ®µł¢µäÅĶ”ŗ’╝īĶ»ĘÕÆ©Ķ»óõĖōõĖÜõ║║ÕŻ½ŃĆéĶ®│Ķ”ŗ
ķå½ÕŁĖĶü▓µśÄ ŃĆé
µ┤Šķåŗńö▓ķģ» ÕĢåÕōüÕÉŹ Concerta, Methylin, Ritalin, Medikinet, Equasym XL, Quillivant XR, Metadate, Ritalin LA, Ritalin SR, Apo-Methylphenidate ÕģČõ╗¢ÕÉŹń©▒ Õōīńö▓ķģ»ŃĆüMethylphenidate AHFS /Drugs.com Monograph MedlinePlus a682188 µĀĖÕćåńŗƵ│ü
µćĘÕŁĢÕłåń┤Ü õŠØĶ│┤µĆ¦ ńö¤ńÉå: ńäĪ µłÉńÖ«µĆ¦ ńäĪ ń╗ÖĶŹ»ķĆöÕŠä ÕÅŻµ£Ź, ÕÖ┤ķ£¦, ķØ£Ķäł, ńČōńÜ« ATCńó╝ µ│ĢÕŠŗĶ”Åń»ä
ńö¤ńē®Õł®ńö©Õ║” ~30% (ń»äÕ£Ź: 11ŌĆō52%) ĶĪƵ╝┐ĶøŗńÖĮńĄÉÕÉłńÄć 10ŌĆō33% ĶŹ»ńē®õ╗ŻĶ░ó ĶéØĶć¤ (80%)ńö¤ńē®ÕŹŖĶĪ░µ£¤ 2ŌĆō3 Õ░ŵŚČ[ 1] µÄƵ│äķĆöÕŠæ Õ░┐µČ▓ (90%)
╬▒-Ķŗ»Õ¤║-2-ÕōīÕĢČõ╣ÖķģĖńö▓ķģ»ńøÉķģĖńøÉ
CASÕÅĘ 113-45-1 Y PubChem CID IUPHAR/BPS DrugBank ChemSpider UNII KEGG ChEBI ChEMBL CompTox Dashboard (EPA ) ECHA InfoCard 100.003.662 Õī¢ÕŁ”Õ╝Å C14 H19 NO2 ┬ĘHCl µæ®Õ░öĶ┤©ķćÅ 233.31 g/mol 3Dµ©ĪÕ×ŗ’╝łJSmol ńåöńé╣ 74 ┬░C’╝ł165 ┬░F’╝ē [ 2] µ▓Ėńé╣ 136 ┬░C’╝ł277 ┬░F’╝ē [ 2]
O=C(OC)C(C1CCCCN1)C2=CC=CC=C2
InChI=1S/C14H19NO2/c1-17-14(16)13(11-7-3-2-4-8-11)12-9-5-6-10-15-12/h2-4,7-8,12-13,15H,5-6,9-10H2,1H3
Y Key:DUGOZIWVEXMGBE-UHFFFAOYSA-N
Y
µ┤Šķåŗńö▓ķģ» ’╝łINN ’╝ÜMethylphenidate’╝īń«Ćń¦░MPHµł¢MPD’╝ē’╝īÕĢåÕōüÕÉŹÕł®õ╗¢µ×Ś ’╝łRitalin’╝ēµł¢ń╝ōķćŖķöŁÕĢåÕōüÕÉŹõĖōµ│©ĶŠŠ ’╝łConcerta’╝ē’╝īµś»õĖĆń©«õĖŁµ©×ńź×ńČōń│╗ńĄ▒ĶłłÕź«ÕŖæ ŃĆéÕģČńĄÉµ¦ŗÕÆīĶŚźńÉåĶłćÕ«ēķØ×õ╗¢ÕæĮ ŃĆüÕÅ»ÕŹĪÕøĀ ńøĖõ╝╝’╝īĶāĮµö╣Õ¢äõĮ┐ńö©ĶĆģńÜäµāģńĘÆÕÆīÕ░łµ│©ÕŖø’╝īĶó½µćēńö©µ¢╝µ│©µäÅÕŖøõĖŹĶČ│ķüÄÕŗĢńŚć ’╝łADHD’╝ēŃĆüÕŚ£ńØĪńŚć ŃĆüĶ║üķ¼▒ńŚć ÕÆīµåéķ¼▒ńŚć ńÜäµ▓╗ńÖéŃĆé
õĖ┤Õ║ŖõĮ┐ńö©Õōīńö▓ķģ»ńÜäńøÉķģĖńøÉ’╝łńøÉķģĖÕōīńö▓ķģ»’╝ē’╝īÕģȵ▓╗ń¢Śµ│©µäÅń╝║ķÖĘÕżÜÕŖ©ķÜ£ńóŹńÜäõĮ£ńö©µ£║ÕłČÕ░ÜõĖŹµĖģµźÜŃĆéÕōīńö▓ķģ»Ķó½Ķ«żõĖ║ķĆÜĶ┐ćķś╗µ¢Łń¬üĶ¦”ÕēŹńź×ń╗ÅÕģāÕ»╣ÕÄ╗ńö▓ĶéŠõĖŖĶģ║ń┤Ā ÕÆīÕżÜÕĘ┤Ķā║ ńÜäÕåŹµæäÕÅ¢’╝īõ╗źÕÅŖÕó×ÕŖĀĶ┐Öõ║øÕŹĢĶā║ńē®Ķ┤©ķćŖµöŠĶć│Õż¢ńź×ń╗ÅÕģāķŚ┤ķÜÖŃĆéÕōīńö▓ķģ»µś»Õż¢µČłµŚŗõĮō’╝īÕÅ│µŚŗÕ╝éµ×äõĮōµ»öÕĘ”µŚŗÕ╝éµ×äõĮōµø┤ÕģĘĶŹ»ńÉåµ┤╗µĆ¦ŃĆé[ 3]
µ┤Šķåŗńö▓ķģ»ńÜäÕī¢ÕŁĖµ£Ćń░ĪÕ╝Å ńé║C14 H19 NO2 ŌĆóHClŃĆé Methylphenidate hydrochloride USP µś»õĖĆń©«ńÖĮĶē▓ŃĆüńäĪÕæ│ńÜäńĄÉµÖČ ķ½öŃĆéµ┤Šķåŗńö▓ķģ»ńÜäµ░┤µ║ȵČ▓ õ╗ŗµ¢╝ķģĖµĆ¦ µ¢╝ķ╣╝µĆ¦ õ╣ŗķ¢ōŃĆéµ┤Šķåŗńö▓ķģ»ĶāĮÕ£©ń┤öµ░┤ ÕÅŖńö▓ķåć õĖŁÕ«īÕģ©µ║ČĶ¦Ż’╝øÕ£©õ╣Öķåć õĖŁķā©Õłåµ║ČĶ¦Ż’╝øõ║øÕŠ«µ║ČĶ¦Żµ¢╝µ░»õ╗┐ ÕÆīõĖÖķģ« õĖŁŃĆéMethylphenidate hydrochloride USPńÜäÕłåÕŁÉķćÅ ńé║269.77ŃĆé[ 4]
µ┤Šķåŗńö▓ķģ»Õ£©1948Õ╣┤ńö▒CIBA ’╝łÕŠīĶó½Ķ½ŠĶÅ»Õģ¼ÕÅĖ µöČĶ│╝’╝ēńĀöÕłČ’╝īĶó½ńö©µ¢╝µ▓╗ńÖéµ│©µäÅÕŖøõĖŹĶČ│ķüÄÕŗĢńŚćĶĆīÕ£©1955Õ╣┤ńŹ▓ÕŠŚFDA ńÜäķŖĘÕö«Ķ©▒ÕÅ»’╝īõĖ”Õ£©õ╣ŗÕŠīńÜäÕ╣ŠÕŹüÕ╣┤ÕåģµłÉńé║ķå½µ▓╗Ķ®▓ńŚćńÜäĶć©Õ║Ŗń¼¼õĖĆńĘÜĶŚźńē®ŃĆé[ 5]
µ┤Šķåŗńö▓ķģ»ńö©µ¢╝ķå½ńÖéÕ¦ŗµ¢╝1960Õ╣┤ŃĆéĶć¬1990Õ╣┤õ╗ŻĶĄĘ’╝īÕż¦ń£ŠķĆɵ╝ĖµÄźÕÅŚADHDńÜäĶ©║µ¢Ę’╝īµ¢╝µś»µŁżĶŚźńē®ńÜäĶÖĢµ¢╣µŚźńøŖÕó×ÕŖĀŃĆéÕ£©2007-2012Õ╣┤ķ¢ō’╝īĶŗ▒Õ£ŗńÜäµ┤Šķåŗńö▓ķģ»ĶÖĢµ¢╣Õó×ÕŖĀõ║å50%ŃĆé[ 6] [ 7] [ 8] [ 9]
µ│©µäÅÕŖøõĖŹĶČ│ķüÄÕŗĢńŚćńÜäńŚģÕøĀÕÅ»ĶāĮĶłćÕżÜÕĘ┤Ķā║ ŃĆüÕÄ╗ńö▓ĶģÄõĖŖĶģ║ń┤Ā ÕÆīń®Ćµ░©ķģĖ µ£ēķŚ£ŃĆéķĆÖõ║øńē®Ķ│¬Õå│Õ«Üõ║åÕż¦Ķģ”ńÜäĶ欵łæń┤äµØ¤ÕŖ¤ĶāĮ’╝īõĖ”ÕĮ▒ķ¤┐ÕĆŗõ║║ńÜäµ│©µäÅÕŖø ŃĆüµÄ¦ÕłČÕŖøŃĆüĶĪīńé║ ŃĆüÕŗĢµ®¤ ÕÆīÕ¤ĘĶĪīĶāĮÕŖøŃĆéµ┤Šķåŗńö▓ķģ»ńÜäõĮ£ńö©õŠ┐µś»µŖæÕłČÕÄ╗ńö▓ĶģÄõĖŖĶģ║ń┤ĀÕÆīÕżÜÕĘ┤Ķā║ńÜäÕåŹÕø×µöČ’╝īõĮ┐ķĆÖõ║øńź×ńČōÕé│ķü×ńē®Ķ│¬ ńÜäµ┐āÕ║”ÕÆīÕ╝║Õ║”Õż¦Õ╣ģµÅÉķ½śŃĆéµ┤Šķåŗńö▓ķģ»ÕÆīÕÅżµ¤»ķ╣╝ Õ£©ńĄÉµ¦ŗÕÆīĶŚźńÉåõĖŖµ£ēńøĖõ╝╝õ╣ŗĶÖĢ’╝īõĮåµ┤Šķåŗńö▓ķģ»ńÜäµĢłÕŖøõĮĵ¢╝ÕŠīĶĆģ’╝īĶŚźµĢłķĢʵ¢╝ÕŠīĶĆģŃĆé[ 10] [ 11] [ 12] [ 13] [ 14] 5HT1A ÕÅŚķ½öĶłłÕź«ÕŖæŃĆé[ 15]
ķĆÖõĖĆĶŚźÕōüÕ£©Õģ©ńÉāń»äÕ£ŹÕåģÕ¤║µ£¼ķāĮÕÅŚÕł░õĖŹÕÉīń©ŗÕ║”ńÜäń«ĪÕłČŃĆé
µĢ┤ķ½öĶĆīĶ©Ć’╝īµ┤Šķåŗńö▓ķģ»ĶłćÕÉīńé║õĖŁµ©×ńź×ńČōĶłłÕź«ÕŖæńÜäÕ«ēķØ×õ╗¢ÕæĮ ńĄÉµ¦ŗõĖŖńøĖõ╝╝’╝īõĮåµ┤Šķåŗńö▓ķģ»ÕÆīÕ«ēķØ×õ╗¢ÕæĮõĖŹÕÉī’╝īõĖŹÕ«╣µśōµłÉńÖ«ŃĆé
ĶĆīńĀöń®ČķĪ»ńż║’╝ÜÕ░Źµ▓Ƶ£ēADHDńÜäõĖĆĶł¼õ║║õŠåĶ¬¬’╝īÕżÜÕŬĶāĮń©ŹÕŠ«ńĖ«ń¤ŁÕÅŹµćēµÖéķ¢ō’╝īÕ░Źµ¢╝ĶĆāĶ®”ķ£ĆĶ”üńÜäĶżćķø£Ķ©śµåČŃĆüÕ¤ĘĶĪīÕŖ¤ĶāĮõĖ”ńäĪÕ╣½ÕŖ®’╝īµ▓Ƶ£ēõĖĆĶł¼Ķ¬Źńé║ŃĆīńź×ńČōõ┐āķĆ▓ŃĆŹńÜäµĢłµ×£’╝īÕøĀµŁżÕ░ŹĶĆāĶ®”µłÉńĖŠõ╣¤µ▓Īµ£ēµśŠĶæŚńÜäÕĮ▒ÕōŹŃĆé
µ┤Šķåŗńö▓ķģ»µś»õĖĆń©«ÕĖĖĶ”ŗńÜäĶłłÕź«ÕŖæķĪ×ńē®’╝īĶŚźńÉåµś»ķś╗µ¢ĘÕÄ╗ńö▓ĶģÄõĖŖĶģ║ń┤ĀÕÆīÕżÜÕĘ┤Ķā║ńÜäÕø×µöČŃĆéÕ«āµēĆĶāĮńöóńö¤ńÜäµĢłµ×£Õīģµŗ¼Õó×ÕŖĀõĖ”ńČŁµīüĶŁ”µāĢµĆ¦ŃĆüµŖĄµŖŚń¢▓Õŗ×ĶłćµÅÉÕŹćµ│©µäÅÕŖøŃĆéµ┤Šķåŗńö▓ķģ»ńÜäń¤Łµ£¤µĢłńøŖĶłćµłÉµ£¼µĢłńøŖÕĘ▓ńČōńó║ń½ŗŃĆéµ┤Šķåŗńö▓ķģ»µ£¬Ķó½Ķ©▒ÕÅ»ńö©µ¢╝6µŁ▓õ╗źõĖŗńÜäÕģÆń½źŃĆéµ┤Šķåŗńö▓ķģ»õ╣¤ÕÅ»ĶāĮńö©µ¢╝µ▓╗ńÖéķØ×ķü®µćēńŚć’╝īõŠŗÕ”éĶ║üķāüńŚć ĶłćķćŹÕ║”µåéķ¼▒ńŚć ŃĆé
µĀĖńŻüÕģ▒µī» ńÜäÕģāÕłåµ×ÉĶłćń│╗ńĄ▒ńČ£Ķ┐░ĶĪ©µśÄ’╝īķĢʵ£¤õĮ┐ńö©ADHDĶłłÕź«ÕŖæ’╝łńē╣Õł½µś»Õ«ēķØ×õ╗¢ÕæĮĶłćµ┤Šķåŗńö▓ķģ»’╝ēÕÅ»õ╗źµĖøÕ░æADHDÕÅŚĶ®”ĶĆģńÜäÕż¦Ķģ”õĖŁńÜäńĄÉµ¦ŗńĢ░ÕĖĖĶłćÕŖ¤ĶāĮńĢ░ÕĖĖŃĆ鵣żÕż¢’╝īĶć©Õ║ŖĶłłÕź«ÕŖæńĀöń®ČńÜäĶ®ĢĶ½¢ÕĘ▓ńó║Õ«Üõ║åķĢʵ£¤ķĆŻń║īõĮ┐ńö©ADHDĶłłÕź«ÕŖæÕ░ŹADHDµéŻĶĆģńÜäÕ«ēÕģ©µĆ¦Ķłćµ£ēµĢłµĆ¦’╝īķĢʵ£¤µēƵīćńÜäµÖéķ¢ōķĢĘķüöµĢĖÕ╣┤ŃĆéńäČĶĆī’╝īµł¬Ķć│2015Õ╣┤11µ£ł’╝īµ┤Šķåŗńö▓ķģ»Õ░ŹµéŻĶĆģńÜäADHDńŚćńŗĆĶłćńö¤µ┤╗ÕōüĶ│¬ńÜäµö╣Õ¢äńÜäń▓Šńó║Õ║”õ╗ŹõĖŹµśÄńó║ŃĆé
µ┤Šķåŗńö▓ķģ»Ķó½ńŠÄÕ£ŗķŻ¤ÕōüĶłćĶŚźÕōüńøŻńØŻń«ĪńÉåÕ▒Ć ’╝łFDA’╝ēµē╣Õćåńö©µ¢╝µ▓╗ńÖéµ│©µäÅÕŖøõĖŹĶČ│ķüÄÕŗĢńŚćŃĆéĶłćĶĪīńé║õ┐«µŁŻ Ķ¬Źń¤źĶĪīńé║ńÖéµ│Ģ ķģŹÕÉłµ▓╗ńÖéµĢłµ×£µø┤õĮ│ŃĆéĶŚźńē®ÕŖæķćÅńÜäÕĆŗķ½öÕĘ«ńĢ░ÕŠłÕż¦’╝īÕøĀµŁżõĮ┐ńö©ńÜäÕŖæķćÅÕ┐ģķĀłń▓Šńó║ŃĆé
ADHDńĢČÕēŹńÜäńŚģńÉ嵩ĪÕ×ŗĶĪ©µśÄ’╝īADHDĶłćÕż¦Ķģ”õĖŁķā©Õłåńź×ńČōÕé│ķü×ńē®Ķ│¬ ń│╗ńĄ▒’╝łÕ░żÕģȵś»µČēÕÅŖÕżÜÕĘ┤Ķā║ ĶłćÕÄ╗ńö▓ĶģÄõĖŖĶģ║ń┤Ā ńÜä’╝ēõĖŁńÜäÕŖ¤ĶāĮķÜ£ńżÖµ£ēķŚ£ŃĆéµ┤Šķåŗńö▓ķģ»ĶłćÕ«ēķØ×õ╗¢ÕæĮķĆÖõĖĆķĪ×ńÜäń▓Šńź×ĶłłÕź«ÕŖæĶāĮÕżĀÕó×ÕŖĀķĆÖõ║øń│╗ńĄ▒õĖŁńÜäńź×ńČōķü║Õé│Õ£░µ┤╗µĆ¦’╝īÕøĀĶĆīÕÅ»ĶāĮµ£ēµĢłÕ£░µ▓╗ńÖéADHDŃĆéń║”70%ńÜäõĮ┐ńö©ĶĆģĶāĮµö╣Õ¢äADHDńÜäńŚćńŖČŃĆéõĮ┐ńö©Õōīńö▓ķģ»ńÜäADHDµéŻÕä┐ķĆÜÕĖĖĶāĮõĖÄÕÉīķŠäõ║║õĖÄÕ«ČÕ║ŁµłÉÕæśÕ╗║ń½ŗµø┤ÕźĮńÜäÕģ│ń│╗’╝īÕ£©ÕŁ”µĀĪĶĪ©ńÄ░µø┤ÕźĮ’╝īõĖöµø┤Õ░æÕłåÕ┐āµł¢Õå▓ÕŖ©’╝īĶāĮõ┐صīüµø┤õ╣ģńÜäµ│©µäÅÕŖøŃĆéADHDµéŻĶĆģńÜäĶŹ»ńē®µ╗źńö©Õż▒ÕĖĖ ÕÅ»ĶāĮµĆ¦Õó×ÕŖĀ’╝īĶĆīÕł║µ┐ƵƦĶŹ»ńē®ĶāĮķÖŹõĮÄĶ┐Öń¦ŹķŻÄķÖ®ŃĆé
ÕŚ£ńØĪńŚćµś»õĖĆń¦ŹµģóµĆ¦ńØĪń£ĀķÜ£ńóŹ ’╝īõ╝ÜÕ£©ńÖĮÕż®Õć║ńÅŠķÜŠõ╗źµŖæÕłČńÜäÕø░ÕĆ”’╝īõ╗źÕÅŖń¬üńäČõ║¦ńö¤ńÜäÕø░µäÅ’╝īõĖ╗Ķ”üķ£ĆĶ”üÕģ┤ÕźŗÕēéõ║łõ╗źµ▓╗ń¢ŚŃĆéÕōīńö▓ķģ»Ķó½Ķ«żõĖ║µ£ēÕŖ®õ║ĵÅÉÕŹćĶ¦ēķåÆÕ║”ŃĆüĶŁ”µāĢµĆ¦õĖÄĶĪīõĖ║ĶĪ©ńÄ░ŃĆé[ 16] ÕżÜķćŹńØĪń£ĀµĮ£õ╝ŵ£¤Ķ»Ģķ¬ī [ 17]
Õōīńö▓ķģ»õ╣¤ÕÅ»ĶāĮńö©õ║ĵ▓╗ń¢Śķ揵ƦµŖæķāüķÜ£ńóŹ ŃĆéÕ«āÕÅ»õ╗źķÖŹõĮÄõĖŁķŻÄ ŃĆüńÖīńŚćõĖÄHIVķś│µĆ¦µéŻĶĆģńÜäµŖæķāüµ░┤Õ╣│ŃĆéńäČĶĆī’╝īńö©Õģ┤ÕźŗÕēéµ▓╗ń¢ŚĶĆÉÕÅŚµĆ¦µŖæķāüńŚćńÜäÕüܵ│ĢÕģʵ£ēõ║ēĶ««ŃĆéÕ£©ĶĆüõ║║õĖÄńŚģõ║║Ķ║½õĖŖ’╝īÕģ┤ÕźŗÕēéÕÅ»ĶāĮµ»öõĖēńÄ»ń▒╗µŖŚµŖæķāüĶŹ» Õģʵ£ēµø┤Õ░æńÜäÕē»õĮ£ńö©ŃĆéÕ»╣õ║ÄńÖīńŚćµÖܵ£¤µéŻĶĆģ’╝īÕōīńö▓ķģ»ÕÅ»ńö©õ║ĵŖĄµŖŚķĖ”ńēćń▒╗ĶŹ»ńē® Õ»╝Ķć┤ńÜäÕŚ£ńØĪ’╝īµ▓╗ń¢ŚµŖæķāüńŚćõĖĵö╣Õ¢äĶ«żń¤źÕŖ¤ĶāĮŃĆé
2015Õ╣┤’╝īÕ»╣ķ½śĶ┤©ķćÅõĖ┤Õ║ŖĶ»Ģķ¬īĶ┐øĶĪīńÜäĶŹ¤ĶÉāÕłåµ×É õĖÄń│╗ń╗¤Ķ»äõ╗Ę õĖŁÕÅæńÄ░’╝īÕ»╣õ║ÄÕüźÕ║ʵłÉõ║║ĶĆīĶ©Ć’╝īµ▓╗ń¢ŚÕēéķćÅńÜäĶŗ»õĖÖĶā║õĖÄÕōīńö▓ķģ»ÕÅ»õ╗źķĆĀµłÉĶ«żń¤źĶāĮÕŖø’╝łÕīģµŗ¼ÕĘźõĮ£Ķ«░Õ┐å ŃĆüµāģµÖ»Ķ«░Õ┐å ÕÆīµŖæÕłČµÄ¦ÕłČĶ»Ģķ¬ī ’╝ēĶĮ╗ÕŠ«õĮåµśÄńĪ«ńÜäµö╣Õ¢ä’╝īÕģČõĮ£ńö©µ£║ÕłČõĖ║ķŚ┤µÄźµ┐Ƶ┤╗ÕēŹķóØÕÅČńÜ«Õ▒é ńÜäÕżÜÕĘ┤Ķā║ÕÅŚõĮōD1 õĖÄĶéŠõĖŖĶģ║ńÜ«Ķ┤©ÕÅŚõĮō╬▒2ŃĆéÕōīńö▓ķģ»õĖÄÕģČõ╗¢ADHDÕģ┤ÕźŗÕēéõ╣¤ÕÅ»õ╗źµÅÉÕŹćõ╗╗ÕŖĪńÜäń¬üÕć║µĆ¦õĖÄÕó×Õ╝║ÕöżĶĄĘŃĆéÕōīńö▓ķģ»ķĆÜĶ┐ćµŖæÕłČÕåŹµæäÕÅ¢ õĖŁµ×óńź×ń╗Åń│╗ń╗¤õĖŁńÜäÕżÜÕĘ┤Ķā║µØźÕó×ÕŖĀõ║║ńÜäĶĆÉÕŖøõĖÄõĖōµ│©Õ║”ŃĆéÕ«ēķØ×õ╗¢ÕæĮõĖÄÕōīńö▓ķģ»Ķ┐ÖõĖĆń▒╗ĶŹ»ńē®ÕÅ»õ╗źµÅÉÕŹćÕ£©µē¦ĶĪīµ×»ńćźõĖÄÕø░ķÜŠõ╗╗ÕŖĪµŚČńÜäĶĪ©ńÄ░’╝īÕ╣ČĶ󽵤Éõ║øÕŁ”ńö¤ńö©õ║ÄÕŁ”õ╣ĀõĖÄĶĆāĶ»ĢńÜäĶŠģÕŖ®ŃĆéÕ¤║õ║ÄĶ欵łæµŖźÕæŖńÜäÕ»╣Õģ┤ÕźŗÕēéĶ┐ØĶ¦äõĮ┐ńö©ńÜäĶ░āµ¤źńĀöń®ČµśŠńż║’╝īµÅÉÕŹćĶĪ©ńÄ░ĶĆīķØ×ńö©õ║ÄÕ©▒õ╣É’╝īµś»ÕŁ”ńö¤õĮ┐ńö©Õģ┤ÕźŗÕēéńÜäõĖ╗Ķ”üÕĤÕøĀŃĆéõĖÄÕ«ēķØ×õ╗¢ÕæĮÕÆīÕ«ēķØ×õ╗¢ÕæĮķģ«ń▒╗õ╝╝’╝īķ½śõ║ĵ▓╗ń¢ŚÕēéķćÅńÜäÕōīńö▓ķģ»ÕÅ»ĶāĮÕ»╣ÕĘźõĮ£Ķ«░Õ┐åõĖÄń«ĪµÄ¦ĶāĮÕŖø µ£ēĶ┤¤ķØóÕĮ▒ÕōŹ’╝īÕÉīµŚČÕż¦ÕēéķćÅńÜäÕōīńö▓ķģ»Ķ┐śÕÅ»ĶāĮµŹ¤Õ«│Ķ┐ÉÕŖ©ĶāĮÕŖø’╝īÕ”éÕ»╝Ķć┤µ©¬ń║╣Ķéīµ║ČĶ¦ŻńŚć õĖÄõĖŁµÜæŃĆé
Õōīńö▓ķģ»µ£ēµŚČĶó½ÕŁ”ńö¤ńö©µØźÕó×Õ╝║ń▓Šńź×ĶāĮÕŖø’╝īÕĖ«ÕŖ®ķøåõĖŁµ│©µäÅÕŖøõĖÄÕĖ«ÕŖ®ÕŁ”õ╣ĀŃĆé
ńö¤ńē®õ╝”ńÉåÕŁ”õĖōÕ«Čń║”ń┐░┬ĘÕōłķćīµ¢»
Barbara Sahakianń¦░Ķ┐Öń¦ŹõĮ┐ńö©Õł®õ╗¢µ×ŚńÜäµ¢╣Õ╝ÅÕÅ»ĶāĮÕ»╝Ķć┤ÕŁ”ńö¤Õ£©ĶĆāĶ»ĢõĖŁµŗźµ£ēõĖŹÕģ¼Õ╣│ńÜäõ╝śÕŖ┐’╝īµ£Ćń╗łÕ»╝Ķć┤Õż¦ÕŁ”ÕÅ»ĶāĮõ╝ÜĶĆāĶÖæĶ”üµ▒éÕŁ”ńö¤µÅÉõŠøÕ░┐µČ▓µĀʵ£¼õ╗źĶ┐øĶĪīĶŹ»ńē®µŻĆµĄŗŃĆé
õ╗źõĖŗµāģÕåĄµćēńČōķå½ÕĖ½Ķ®Ģõ╝░ÕŠīÕåŹõĮ┐ńö©Õōīńö▓ķģ»’╝Ü
ńŠÄÕøĮFDAÕ»╣Õōīńö▓ķģ»ńÜäµĆĆÕŁĢÕłåń║¦õĖ║C’╝īÕ╗║Ķ««Õź│µĆ¦ÕŬգ©µĢłńö©ĶČģĶ┐ćµĮ£Õ£©ķŻÄķÖ®µŚČõĮ┐ńö©ŃĆéĶ┐śµ▓Īµ£ēĶČ│Õż¤ńÜäÕŖ©ńē®Õ«×ķ¬īÕÆīõ║║ń▒╗ńĀöń®ČĶČ│õ╗źĶ»┤µśÄÕōīńö▓ķģ»Õ»╣ĶāÄÕä┐ÕÅæĶé▓ńÜäõĮ£ńö©ŃĆéĶć│2007Õ╣┤’╝īÕ«×Ķ»üµ¢ćńī«õĖŁÕģ▒ÕīģÕɽµØźĶć¬3õĖ¬Õ«×Ķ»üńĀöń®ČńÜä63µØĪõ║¦ÕēŹµÄźĶ¦”Õōīńö▓ķģ»ńÜäµĪłõŠŗŃĆé
Õōīńö▓ķģ»µś»õĖĆń¦ŹĶŗ»ÕōīÕĢČĶĪŹńö¤ńē®ŃĆéÕ«āõĖÄÕä┐ĶīČķģÜĶā║ÕÆīĶŗ»õ╣ÖĶā║µ£ēńøĖÕÉīńÜäÕ¤║µ£¼ń╗ōµ×äŃĆé
Õōīńö▓ķģ»õĖ╗Ķ”üÕģģÕĮōÕÄ╗ńö▓ĶģÄõĖŖĶģ║ń┤Ā-ÕżÜÕĘ┤Ķā║ÕåŹµöØÕÅ¢µŖæÕłČÕŖæ (NDRI)’╝īõĖĆĶł¼µ£ēĶ░āµĢ┤ÕżÜÕĘ┤Ķā║µ░┤Õ╣│Õ£©ĶŠāÕ░Åń©ŗÕ║”õĖŖõ╣¤ÕĮ▒ÕōŹÕÄ╗ńö▓ĶéŠõĖŖĶģ║ń┤ĀńÜäõĮ£ńö©ŃĆé[ 21] ÕÅżµ¤»ńó▒ ńøĖõ╝╝’╝īÕōīńö▓ķģ»õĖÄÕżÜÕĘ┤Ķā║Ķ┐ÉĶĮ¼õĮō (DAT)ÕÆīÕÄ╗ńö▓ĶéŠõĖŖĶģ║ń┤ĀĶ┐ÉĶĮ¼õĮō (NET)ń╗ōÕÉłÕ╣ȵŖæÕłČÕģČõĮ£ńö©ŃĆé[ 22]
µŁżÕż¢’╝īÕōīńö▓ķģ»Ķó½Ķ«żõĖ║µś»õĖĆń¦ŹķćŖµöŠÕēé’╝īķĆÜĶ┐ćµÅÉÕŹćÕżÜÕĘ┤Ķā║ÕÆīÕÄ╗ńö▓ĶéŠõĖŖĶģ║ń┤ĀńÜäķćŖµöŠ’╝īõĮåõĮ£ńö©õĖŹÕÅŖÕ«ēķØ×õ╗¢ÕæĮ ŃĆé[ 23] [ 24] [ 25] [ 26] [ 27]
Õōīńö▓ķģ»Õģʵ£ēÕżÜÕĘ┤Ķā║Ķ┐ÉĶĮ¼õĮōÕÆīÕÄ╗ńö▓ĶéŠõĖŖĶģ║ń┤ĀĶ┐ÉĶĮ¼õĮōńÜäõ║▓ÕÆīÕŖø’╝īÕ«āńÜäÕÅ│µŚŗÕ»╣µśĀõĮōÕ»╣ÕÄ╗ńö▓ĶéŠõĖŖĶģ║ń┤ĀĶ┐ÉĶĮ¼õĮōµśŠńż║õ║åÕ╝║ńāłńÜäõ║▓ÕÉłÕŖ┐ŃĆéÕĘ”ÕÅ│õĖżń¦ŹÕ»╣µśĀõĮōķāĮµśŠńż║õ║åÕ»╣5HT1A ÕÆī5HT2B ÕŁÉń▒╗Õ×ŗńÜä5-ńŠ¤Ķē▓Ķā║ÕÅŚõĮō ńÜäõ║▓ÕÆīÕŖø’╝īÕ░Įń«Īµ▓Īµ£ēĶ¦éÕ»¤Õł░ÕÆī5-ńŠ¤Ķē▓Ķā║ĶĮ¼Ķ┐ÉõĮō ńø┤µÄźńÜäń╗ōÕÉłŃĆé[ 20]
Õōīńö▓ķģ»ÕÅ»ĶāĮõ╣¤õ╝Üõ║¦ńö¤õ┐صŖżńź×ń╗ÅńÜäõĮ£ńö©ŃĆé[ 28]
ÕÅ│µŚŗÕ»╣µśĀõĮōµ»öÕĘ”µŚŗÕ»╣µśĀõĮōµĢłµ×£ńöÜõĮ│[ 21] [õŠåµ║ÉĶ½ŗµ▒é]
ÕÅŻµ£ŹÕōīńö▓ķģ»ĶāĮĶŠŠÕł░11-52%ńÜäńö¤ńē®Õł®ńö©Õ║”’╝īõĖƵ¼ĪµĆ¦ķćŖµöŠ’╝łÕ”éÕł®õ╗¢µ×Ś’╝ēÕģČÕ│░ÕĆ╝µĢłµ×£ĶāĮµīüń╗Ł2-4Õ░ŵŚČ’╝īĶĆīń╝ōķćŖ’╝łÕ”éÕł®õ╗¢µ×ŚSR’╝ēÕÅ»µīüń╗Ł3-8Õ░ŵŚČ’╝īµł¢Õ╗ČķĢ┐ķćŖµöŠńēćÕēéÕÅ»µīüń╗Ł8-12Õ░ŵŚČ’╝łÕ”éConcerta’╝ēŃĆéÕōīńö▓ķģ»ńÜäÕŹŖĶĪ░µ£¤õĖ║2-3Õ░ŵŚČ’╝īÕÅ¢Õå│õ║ÄõĖ¬õĮō’╝īµ£ŹĶŹ»ń║”2Õ░ŵŚČÕÉÄĶĪƵĄåµĄōÕ║”ĶŠŠÕł░Õ│░ÕĆ╝ŃĆé
ÕÅŻµ£Źń╗ÖĶŹ»µŚČ’╝īÕōīńö▓ķģ»ńÜäÕÅ│µŚŗÕÉīÕłåÕ╝éµ×äõĮōńÜäńö¤ńē®Õł®ńö©Õ║”µ»öÕģČÕĘ”µŚŗÕÉīÕłåÕ╝éµ×äõĮōµø┤ķ½ś’╝īõĖöµś»Õōīńö▓ķģ»Õż¢µČłµŚŗµĘĘÕÉłńē® õĖŁĶĄĘń▓Šńź×µ┤╗µĆ¦õĮ£ńö©ńÜäõĖ╗Ķ”üńē®Ķ┤©ŃĆé
õĖÄķóäĶ«ĪńøĖÕÅŹ’╝īÕ£©ķżÉõĖŁµ£Źńö©Õōīńö▓ķģ»ÕÅ»ÕŖĀÕ┐½ÕÉĖµöČŃĆé
Õōīńö▓ķģ»Õ£©ńŠ¦ķģĖķģ»ķģČ1 Õł®õ╗¢µ×ŚķģĖ
Õōīńö▓ķģ»ķĆÜÕĖĖµ£ēķ½śńÜäĶĆÉÕÅŚķćÅŃĆéĶ¦éÕ»¤Õł░µ£ĆÕĖĖĶ¦üńÜäÕē»õĮ£ńö©Õīģµŗ¼ķŻ¤µ¼▓õĖŹµī»ŃĆüÕÅŻÕ╣▓ŃĆüńä”ĶÖæ/ń┤¦Õ╝ĀŃĆüµüČÕ┐āõĖÄÕż▒ń£ĀŃĆéĶāāĶéĀķüōńÜäõĖŹĶē»ÕÅŹÕ║öÕÅ»Õīģµŗ¼Ķģ╣ķā©ń¢╝ńŚøÕÆīõĮōķćŹÕćÅĶĮ╗ŃĆéńź×ń╗Åń│╗ń╗¤ńÜäÕē»õĮ£ńö©ÕÅ»Õīģµŗ¼ÕØÉń½ŗõĖŹÕ«ēŃĆüµśōµĆÆŃĆüĶ┐ÉÕŖ©ķÜ£ńóŹ Õ┐āĶĘ│Ķ┐ćķƤ ŃĆéń£╝ń¦æµ¢╣ķØóńÜäõĖŹĶē»ÕÅŹÕ║öÕÅ»ĶāĮÕīģµŗ¼Ķ¦åÕŖøµ©Īń│ŖÕÆīÕ╣▓ń£╝’╝īńĮĢĶ¦üÕżŹĶ¦åõĖÄń×│ÕŁöµē®Õż¦ŃĆéÕģČõ╗¢ńÜäÕē»õĮ£ńö©ÕÅ»ĶāĮÕīģµŗ¼µŖæķāüŃĆüµāģń╗¬õĖŹń©│ŃĆüńź×Õ┐ŚõĖŹµĖģÕÆīńŻ©ńēÖŃĆéÕĖĖĶ¦üÕżÜµ▒Ś’╝īńĮĢĶ¦üĶāĖńŚøŃĆé
µ£ēķā©ÕłåĶ»üµŹ«ĶĪ©µśÄķĢ┐µ£¤ńö©ĶŹ»ńÜäÕä┐ń½źÕć║ńÄ░õ║åńĢźÕŠ«ńÜäńö¤ķĢ┐ķƤÕ║”õĖŗķÖŹ’╝īõĮåµ▓Īµ£ēÕÅæńÄ░ÕøĀµ×£Õģ│ń│╗’╝īõĖöõĖŗķÖŹńÜäµāģÕåĄµ▓Īµ£ēķĢ┐µ£¤ÕŁśÕ£©ŃĆéĶČģµĢÅÕÅŹÕ║ö ’╝łÕīģµŗ¼ńÜ«ń¢╣ ŃĆüĶŹ©ķ║╗ń¢╣ ÕÆīÕÅæńā¦’╝ēµŚČµ£ēµŖźķüōŃĆé
Õōīńö▓ķģ»ÕÅ»õ╗źõĮ┐ń▓Šńź×ńŚģµéŻĶĆģńÜäń▓Šńź×ńŚģµüČÕī¢’╝īõĖöÕ£©ķØ×ÕĖĖńĮĢĶ¦üńÜäµāģÕåĄõĖŗÕŻջ╝Ķć┤µ¢░ńÜäń▓Šńź×ńŚģńŚćńŖČńÜäÕć║ńÄ░ŃĆéńö▒õ║ĵŁżĶŹ»ńē®Õ»╣ńŗéĶ║üõĖÄĶĮ╗Õ║”ńŗéĶ║ü µ£ēµĮ£Õ£©ńÜäĶ»▒ÕÅæµĆ¦’╝īÕ£©ńö©õ║ÄĶ║üķāüńŚć µéŻĶĆģµŚČÕ║öńē╣Õł½Õ░ÅÕ┐āŃĆéõ╣¤µ£ēķØ×ÕĖĖńĮĢĶ¦üńÜäÕ╝ĢÕÅæĶ欵ØĆÕ┐ĄÕż┤ńÜäµŖźķüō’╝īõĮåõĖŹĶČ│õ╗źÕ╗║ń½ŗÕøĀµ×£Ķüöń│╗ŃĆé
ÕüČÕ░öµ£ēÕć║ĶĪĆńÜäµŖźÕæŖŃĆéÕŠłÕ░æµ£ēÕ»╝Ķć┤µĆ¦µ¼▓ķÜ£ńóŹŃĆüÕ«ÜÕÉæÕŖøķÜ£ńóŹõĖÄÕ╣╗Ķ¦ēńÜäµŖźķüōŃĆéķś┤ĶīÄÕ╝éÕĖĖÕŗāĶĄĘ µś»ÕģČÕÅ»ĶāĮÕ»╝Ķć┤ńÜäķØ×ÕĖĖńĮĢĶ¦üõĮåÕÅ»ĶāĮõĖźķćŹńÜäõĖŹĶē»ÕÅŹÕ║öŃĆé
2011Õ╣┤’╝īńö▒FDAÕ¦öµēśńÜäńĀöń®ČĶĪ©µśÄ’╝īÕ£©Õä┐ń½źõĖĵłÉõ║║õĖŁ’╝īÕōīńö▓ķģ»µł¢ÕģČõ╗¢ADHDÕģ┤ÕźŗÕēéńÜäÕī╗ń¢Śńö©ķĆöõĖŹõ╝ÜÕ»╝Ķć┤õĖźķćŹõĖŹĶē»Õ┐āĶĪĆń«Īõ║ŗõ╗Č’╝łńīصŁ╗’╝īÕ┐āĶäÅńŚģÕÅæõĮ£ÕÆīõĖŁķŻÄ’╝ēŃĆé
ķā©ÕłåõĖŹĶē»ÕÅŹÕ║öÕŬգ©ķĢ┐µ£¤õĮ┐ńö©Õōīńö▓ķģ»µŚČÕć║ńÄ░’╝īµĢģÕ║öńĢÖµäÅńö©ĶŹ»µ£¤ķŚ┤Õć║ńÄ░ńÜäõĖŹĶē»ÕÅŹÕ║öŃĆé[ 29] [ 30] [ 31]
ÕģČõ╗¢ńÜäÕē»õĮ£ńö©Õīģµŗ¼’╝Ü[ 32]
Õōīńö▓ķģ»µĆźµĆ¦Ķ┐ćķćÅńÜäńŚćńŖČõĖ╗Ķ”üµś»ÕøĀõĖ║õĖŁµ×óńź×ń╗Åń│╗ń╗¤ÕÅŚÕł░Ķ┐ćÕ║”Õł║µ┐ĆŃĆéĶ┐Öõ║øńŚćńŖČÕīģµŗ¼’╝ÜÕæĢÕÉÉŃĆüńä”ĶÖæŃĆüķ£ćķóżŃĆüÕÅŹÕ░äõ║óĶ┐ø µ¼ŻÕ┐½ ŃĆüÕ╣╗Ķ¦ēŃĆüĶ░ĄÕ”ä ŃĆüõĖŁµÜæŃĆüÕć║µ▒ŚŃĆüµĮ«ń║óŃĆüÕż┤ńŚøŃĆüÕ┐āĶĘ│Ķ┐ćķƤ ŃĆüÕ┐āÕŠŗÕż▒ÕĖĖ ŃĆüÕ┐āµéĖ ŃĆüķ½śĶĪĆÕÄŗ ŃĆüń×│ÕŁöµē®Õż¦ÕÆīķ╗ÅĶå£ Õ╣▓ńćźŃĆé
õĖźķćŹńÜäĶ┐ćķćÅÕÅ»ĶāĮÕ»╝Ķć┤ķ½śńāŁ’╝łõĖŹõĮÄõ║Ä41.5Ōäā’╝ēŃĆüĶéŠõĖŖĶģ║ń┤ĀĶ┐ćÕżÜŃĆüµāŖÕÄź ŃĆüÕüŵē¦ ŃĆüÕł╗µØ┐ńŚć µ©¬ń║╣Ķéīµ║ČĶ¦ŻńŚć ’╝ēŃĆüµśÅĶ┐Ę ÕÆīÕŠ¬ńÄ»ĶĪ░ń½Ł ŃĆé
µ£ēÕ░åÕōīńö▓ķģ»ńēćÕēéµ│©Õ░äÕģźÕŖ©ĶäēÕÉÄÕ»╝Ķć┤ĶäōĶé┐ ÕÆīÕØŵŁ╗ ńÜäµ»ÆµĆ¦ÕÅŹÕ║öńÜäµŖźķüōŃĆé
Õ”éµ×£õ║łõ╗źķĆéÕĮōńÜäµĢæÕŖ®’╝īÕōīńö▓ķģ»Ķ┐ćķćÅÕŠłÕ░æĶć┤ÕæĮŃĆé
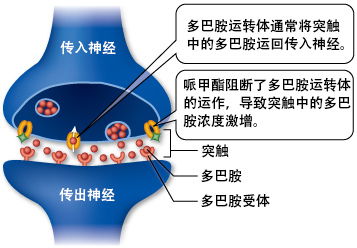
ĶŹ»ńÉåÕŁ”µ¢ćń½ĀĶ«żõĖ║Õōīńö▓ķģ»µś»õĖĆń¦ŹµĢłµ×£ŃĆüµłÉńśŠµĆ¦ŃĆüõŠØĶĄ¢µĆ¦õĖÄĶŗ»õĖÖĶā║ ’╝łÕ«ēķØ×õ╗¢ÕæĮ’╝ēń▒╗õ╝╝ńÜäÕģ┤ÕźŗÕēé’╝īĶĆīĶŗ»õĖÖĶā║ Õ£©ÕÅ»µłÉńśŠĶŹ»ńē®õĖŁÕżäõ║ÄõĖŁńŁēŃĆéÕøĀµŁż’╝īÕĮōµŖŖÕōīńö▓ķģ»ķ½śÕēéķćÅõĮ┐ńö©õĮ£õĖ║Õ©▒õ╣ɵƦĶŹ»ńē®µŚČ’╝īµłÉńśŠõĖÄÕ┐āńÉåõŠØĶĄ¢µś»ÕÅ»ĶāĮńÜäŃĆéÕĮōõĮ┐ńö©ÕēéķćÅÕż¦õ║ÄÕī╗ńö©ÕēéķćŵŚČ’╝īÕģ┤ÕźŗÕēéõĖÄÕģ┤ÕźŗÕēéÕ×ŗń▓Šńź×ńŚģ ╬öFosB Õ£©õ╝ÅķÜöµĀĖ õĖŁńÜäD1Õ×ŗ õĖŁńŁēµ£ēµŻśńź×ń╗ÅÕģā õĖŁĶ┐ćķćÅĶĪ©ĶŠŠŃĆé
Õōīńö▓ķģ»õĮ£õĖ║õĖĆń¦ŹµłÆķÖżńö▓Õ¤║Ķŗ»ńö▓Ķā║ µłÉńśŠĶ┐ćń©ŗõĖŁńÜäµø┐õ╗Żńē®Ķ┤©µŚČĶĪ©ńÄ░Õć║õ║åõĖĆõ║øÕźĮńÜäµĢłµ×£ŃĆéÕōīńö▓ķģ»ÕÆīÕ«ēķØ×õ╗¢ÕæĮÕĘ▓Ķó½ńĀöń®ČõĮ£õĖ║ÕÅ»ÕŹĪÕøĀ µłÉńśŠµ▓╗ń¢ŚõĖŁńÜäÕī¢ÕŁ”µø┐õ╗ŻÕōü’╝īńŠÄµ▓Öķģ« õ╣¤Ķó½õ╗źÕÉīµĀĘńÜäµ¢╣Õ╝Åńö©õĮ£µø┐õ╗ŻĶŹ»ńē®µ▓╗ń¢ŚÕ»╣µĄĘµ┤øÕøĀ ńÜäńö¤ńÉåõŠØĶĄ¢ŃĆéÕģČÕ£©µ▓╗ń¢ŚÕÅ»ÕŹĪÕøĀµł¢Õ┐āńÉå/ńö¤ńÉåµłÉńśŠµ¢╣ķØóńÜäõĮ£ńö©Õ░ܵ£¬Ķ»üÕ«×’╝īĶ┐śµ£ēÕŠģĶ┐øõĖƵŁźńĀöń®ČŃĆé
ÕøĀõĖ║Õōīńö▓ķģ»Õ£©Õż¦ĶäæńÜäÕź¢ĶĄÅń│╗ń╗¤ õĖŁńÜäĶŹ»ńē®µĢłÕ║öÕŖ©ÕŖøÕŁ” õĮ£ńö©’╝łµŖæÕłČÕżÜÕĘ┤Ķā║ÕåŹµæäÕÅ¢’╝ē’╝īÕģČÕģʵ£ēÕ»╝Ķć┤µ¼ŻÕ┐½ ńÜäµĮ£ÕŖøŃĆéÕ£©µ▓╗ń¢ŚÕēéķćÅõĖŗ’╝īADHDÕģ┤ÕźŗÕēéõĖŹõ╝ÜÕģģÕłåµ┐Ƶ┤╗Õź¢ĶĄÅń│╗ń╗¤’╝īµł¢ĶĆģÕź¢ĶĄÅĶĘ»ÕŠä ╬öFosB Õ¤║ÕøĀÕ£©õ╝ÅķÜöµĀĖ õĖŁńÜäD1Õ×ŗ õĖŁńŁēµ£ēµŻśńź×ń╗ÅÕģā õĖŁµīüõ╣ģĶĪ©ĶŠŠŃĆéÕøĀµŁż’╝īÕĮōńö©õ║ÄÕī╗ń¢ŚõĖöķüĄńģ¦Õī╗Õś▒µŚČ’╝īÕōīńö▓ķģ»õĖŹõ╝ܵłÉńśŠŃĆéńäČĶĆī’╝īÕĮōÕōīńö▓ķģ»õ╗źĶČ│Õż¤ķ½śńÜäÕēéķćÅķĆÜĶ┐ćķ½śńö¤ńē®Õł®ńö©Õ║”ńÜäń╗ÖĶŹ»ķĆöÕŠä’╝łÕ”éÕÉĖÕģźµł¢ķØÖĶäēµ│©Õ░ä’╝ēõĮ┐ńö©’╝īÕ░żÕģȵś»ńö©õĮ£Õģ┤ÕźŗÕē鵌Ȓ╝ī╬öFosB õ╝ÜÕ£©õ╝ÅķÜöµĀĖ õĖŁń¦»ń┤»ŃĆéÕøĀµŁż’╝īõĖÄÕģČõ╗¢ÕÅ»µłÉńśŠĶŹ»ńē®õĖƵĀĘ’╝īÕ©▒õ╣Éńö©ķĆöńÜäķ½śÕēéķćÅÕōīńö▓ķģ»µ£Ćń╗łõ╝ÜÕ»╝Ķć┤╬öFosB Õ£©õ╝ÅķÜöµĀĖ õĖŁńÜäD1Õ×ŗ õĖŁńŁēµ£ēµŻśńź×ń╗ÅÕģā õĖŁĶ┐ćķćÅĶĪ©ĶŠŠ’╝īķÜÅÕÉÄÕ╝ĢÕÅæõĖĆń│╗ÕłŚµ┐Ƶ┤╗Õ¤║ÕøĀĶĮ¼ÕĮĢńÜäõ┐ĪÕÅĘĶĮ¼Õ»╝ ’╝īÕ»╝Ķć┤õĖŖńśŠŃĆé
Õōīńö▓ķģ»ÕÅ»õ╗źµŖæÕłČĶ▒åķ”Öń┤ĀµŖŚÕćØĶĪĆÕēé õĖēńÄ»ń▒╗µŖŚķāüĶŹ» ÕÆīķĆēµŗ®µĆ¦5-ńŠ¤Ķē▓Ķā║ÕåŹµæäÕÅ¢µŖæÕłČÕēé ’╝ēńÜäõ╗ŻĶ░óŃĆéÕģ▒ÕÉīń╗ÖĶŹ»ÕÅ»ĶāĮķ£ĆĶ”üĶ░āµĢ┤ÕēéķćÅ’╝īÕÅ»ĶāĮķ£ĆĶ”üńøæµĄŗĶĪƵĄåĶŹ»ńē®µĄōÕ║”µØźĶŠģÕŖ®ŃĆé
[ 35]
ÕĮōÕōīńö▓ķģ»õĖÄõ╣ÖķåćÕģ▒ÕŁśµŚČ’╝īõ╝ÜķĆÜĶ┐ćĶéØõĖŁńÜäķģ»õ║żµŹó ńö¤µłÉõ╗ŻĶ░óńē®Õōīõ╣Öķģ» [ 36] [ 37] ķ½śÕÅżµ¤»ńó▒ [ 38] [ 37]
õĖÄķģÆń▓ŠńÜäµĘĘÕÉłõĮ£ńö©ÕŻջ╝Ķć┤d-Õōīńö▓ķģ»ńÜäĶĪƵĄåµĄōÕ║”õĖŖÕŹćµ£ĆÕżÜ40%ŃĆé
[ 39]
Õōīńö▓ķģ»ńÜäĶéص»ÆµĆ¦ ╬▓ĶéŠõĖŖĶģ║ń┤Āµ┐ĆÕŖ©Õēé [ 40]
ķś┐µēśĶĽĶź┐µ▒Ć ńé║ÕÅ”õĖĆń©«ÕĖĖĶ”ŗńö©µ¢╝µ▓╗ńÖéµ│©µäÅÕŖøõĖŹĶČ│/ķüÄÕŗĢńŚć’╝łAD/HD’╝ēńÜäń¼¼õĖĆńĘÜĶŚźńē®ŃĆé
ńĖ▒ńäČķś┐µēśĶĽĶź┐µ▒ĆĶłćõĖŁµ©×ńź×ńČōĶłłÕź«ÕŖæÕÉīµ©Żńé║µ▓╗ńÖéADHDńÜäń¼¼õĖĆńĘÜĶŚźńē® ’╝īńäČĶĆīÕģČÕ░Źńē╣Õ«ÜńŚćńŗƵö╣Õ¢äńÜäń©ŗÕ║”ÕÅ»ĶāĮĶłćõĖŁµ©×ńź×ńČōĶłłÕź«ÕŖæõĖŹÕÉī’╝łÕģ®ķĪ×ĶŚźńē®ÕÉäµ£ēÕģČķĢĘĶÖĢ’╝ēŃĆéķś┐µēśĶĽĶź┐µ▒ĆÕ£©µö╣Õ¢äķüÄÕŗĢ-ĶĪØÕŗĢńÜäńŚćńŗĆõĖŖ’╝īńĢźÕ䬵¢╝Õōīńö▓ķģ»’╝øÕōīńö▓ķģ»ÕēćÕ£©µö╣Õ¢äÕłåÕ┐āńÜäńŚćńŗĆõĖŖ’╝īńĢźÕ䬵¢╝ķś┐µēśĶĽĶź┐µ▒ĆŃĆé[ 41] [ 42] [ 43]
ĶĆīķś┐µēśĶĽĶź┐µ▒ĆĶłćÕōīńö▓ķģ»õĮĄµ£ŹńÜäĶÖĢµ¢╣Õ░ܵ£¬ńČōńŠÄÕ£ŗķŻ¤ÕōüĶŚźńē®ń«ĪńÉåÕ▒Ć µĀĖÕÅ»’╝īõĮåķå½ÕĖ½µ£āĶ”¢ÕĆŗµĪłńÜäµāģµ│ü’╝łÕ”éÕģ▒ńŚģŃĆüķĀÉÕŠī’╝ēõ╗źķ¢ŗõ╗┐Õ¢«µ©Öńż║Õż¢õĮ┐ńö© ńÜäµ¢╣Õ╝ÅĶÖĢµ¢╣õ╣ŗŃĆé[ 44] [ 45] [ 46] [ 47] [ 48]
Õōīńö▓ķģ»ÕÅ»ĶāĮµ£ēÕøøń¦ŹÕÉīÕłåÕ╝éµ×äõĮō’╝īÕøĀõĖ║ÕģČÕłåÕŁÉÕģʵ£ēõĖżõĖ¬µēŗµĆ¦õĖŁÕ┐ā’╝īÕÅ»ÕłåõĖ║õĖĆÕ»╣ĶĄżÕ╝ÅõĖÄõĖĆÕ»╣ĶŗÅÕ╝ÅÕ╝éµ×äõĮō’╝īÕģČõĖŁÕŬµ£ēÕÅ│-ĶŗÅÕ╝Å-Õōīńö▓ķģ»µ£ēĶŹ»ńē®µ┤╗µĆ¦ŃĆéÕĮōµŁżĶŹ»ÕłÜõĖŖÕĖ鵌Ȓ╝īõ╗źĶĄżÕ╝Å:ĶŗÅÕ╝ŵīē3:1µĘĘÕÉłŃĆéĶĄżÕ╝ÅķØ×Õ»╣µśĀÕ╝éµ×äõĮōõ╣¤µś»ÕŹćÕÄŗĶā║ŃĆé
Õōīńö▓ķģ»ÕÅŖÕģČõĖ╗Ķ”üõ╗ŻĶ░óńē®Õł®õ╗¢µ×ŚķģĖÕŻգ©ĶĪƵĄåŃĆüĶĪƵĖģµł¢Õģ©ĶĪĆõĖŁµŻĆķ¬ī’╝īõ╗źńøæµĄŗńŚģõ║║µś»ÕÉ”µīēÕī╗Õś▒ńö©ĶŹ»’╝īńĪ«Ķ«żÕÅ»ĶāĮńÜäõĖŁµ»ÆĶĆģ’╝īµł¢ÕŹÅÕŖ®ÕēéķćÅĶć┤ÕæĮµŚČńÜäµ│ĢÕī╗Ķ░āµ¤źŃĆé
õ╗źõĖŗĶŚźÕōüµēĆÕɽõ╣ŗµ£ēµĢłµłÉÕłåńÜåńé║Õōīńö▓ķģ»’╝īÕÉäĶć¬Õ£©ĶŚźµĢłÕŗĢÕŖøÕŁĖ õĖŖÕģʵ£ēńøĖÕÉīÕ▒¼µĆ¦’╝øÕ£©ĶŚźńē®õ╗ŻĶ¼ØÕŗĢÕŖøÕŁĖ õĖŖńÜäõĮ£ńö©Õēćµ£ēõ║øÕŠ«ÕĘ«ńĢ░ŃĆé
Õł®õ╗¢µ×Ś’╝łRitalin’╝ē[ 49]
Õł®ķĢĘĶāĮ’╝łRitalin LA’╝ē[ 50]
Õ░łµĆØķüö’╝łConcerta’╝ē[ 51]
Õ«ēõ┐ØńŠÄÕ¢£ķīĀ’╝łApo-Methylphenidate’╝ē[ 52] [ 53] ĶŚźÕōüńÜäÕŖæķćŵćēµÄĪÕĆŗõ║║Õī¢ńÜäµ¢╣Õ╝ÅĶ”¢µéŻĶĆģńÜäÕÅŹµćēÕÅŖķ£ĆĶ”üõŠåµ▒║Õ«Üõ╣ŗŃĆé[ 54]
µ£ē5ŃĆü10ŃĆüÕÆī20 mgńŁēõĖēń©«ÕÅŻµ£ŹķīĀÕŖæÕ×ŗŃĆé[ 55]
õĮ£ńö©µÖéķ¢ō’╝Üń┤ä3.5Õ░ŵÖéŃĆé
ÕżÜµĢĖµéŻĶĆģńÜäÕ╣│ÕØćÕŖæķćÅńé║µ»ÅÕż®ńĖĮÕŖæķćÅ20-30µ»½Õģŗ’╝łmg’╝ē’╝īõĖ”Õ£©õĖĆÕż®õĖŁÕłå2-3µ¼ĪķüöµłÉŃĆéÕ╗║ĶŁ░Õ£©ķżÉÕēŹ30-45ÕłåķÉśÕēŹµ£Źńö©ŃĆéµ£ēõ║øµéŻĶĆģńÜ䵻ŵŚźńĖĮÕŖæķćÅ ÕÅ»ĶāĮķ£ĆĶ”üÕł░ķüö40-60µ»½Õģŗ’╝īõĖ”Õ£©õĖĆÕż®õĖŁÕłå2-3µ¼ĪķüöµłÉŃĆéķÖżõ║åõ╗źõĖŖÕģ®ĶĆģ’╝īµéŻĶĆģńÜ䵻ŵŚźńĖĮÕŖæķćÅń┤äµÄ¦ÕłČÕ£©10-15 µ»½Õģŗ’╝īõĖ”Õ£©õĖĆÕż®õĖŁÕłå2-3µ¼ĪķüöµłÉÕŹ│ÕÅ»ŃĆé
[ 56]
ĶĄĘÕ¦ŗÕŖæķćÅńé║ÕłåÕłźÕ£©µŚ®ķżÉÕÆīÕŹłķżÉÕēŹµ£Źńö©õĖĆķĪå5µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮŃĆéõĖ”Ķ”¢ķ£ĆĶ”ü’╝īõ╗źõĖĆÕĆŗµś¤µ£¤ńé║õĖƵ¼ĪÕŖæķćÅńÜäĶ¬┐µĢ┤ķĆ▒µ£¤’╝īµ»Åµ¼ĪĶ¬┐µĢ┤ńÜäÕ╣ģÕ║”ńé║5µł¢10µ»½Õģŗ’╝łmg’╝ēŃĆéńäĪĶ½¢ÕłåÕ╣Šµ¼Īµ£Źńö©’╝īµ»ÅÕż®ńÜäńĖĮÕŖæķćÅõĖŹÕ╗║ĶŁ░ĶČģķüÄ60µ»½ÕģŗŃĆé
[ 57]
Õł®ķĢĘĶāĮ Õģ▒µ£ē10ŃĆü20ŃĆü30ŃĆü40ÕÆī60µ»½Õģŗõ║öń©«ÕŖæÕ×ŗ’╝īÕłåÕłźĶłćµ»ÅµŚźµ£Źńö©Õģ®µ¼ĪńÜäń¤ŁµĢłÕł®õ╗¢ĶāĮõ╣ŗ5ŃĆü10ŃĆü15ŃĆü20ŃĆüÕÆī30 µ»½Õģŗ’╝łmg’╝ēÕŖæÕ×ŗńøĖÕ░ŹµćēŃĆé[ 58]
Õ╗║ĶŁ░ĶĄĘÕ¦ŗÕŖæķćÅńé║µ»ÅÕż®õĖĆń▓Æ20µ»½ÕģŗńÜäÕł®ķĢĘĶāĮŃĆéńäČĶĆīķå½ÕĖ½ÕÅ»õŠØńģ¦Ķć©Õ║ŖÕłżµ¢ĘÕ░ćĶĄĘÕ¦ŗÕŖæķćÅķÖŹĶć│µ»ÅÕż®õĖĆń▓Æ10µ»½ÕģŗńÜäÕł®ķĢĘĶāĮŃĆéÕŠĆÕŠīńÜ䵌źÕŁÉõĖŁ’╝īÕŖæķćÅńÜäĶ¬┐µĢ┤Õ╗║ĶŁ░õ╗źõĖĆÕæ©Ķ¬┐µĢ┤10µ»½Õģŗ’╝łmg’╝ēńé║µ║¢ŃĆéÕł®ķĢĘĶāĮńÜäÕŖæķćÅÕÅ»õŠØńģ¦µéŻĶĆģÕ░ŹĶŚźńē®ńÜäĶĆÉÕÅŚµĆ¦ÕÅŖńŚćńŗƵö╣Õ¢äńÜäń©ŗÕ║”Õ░ćÕŖæķćÅõ╗źõĖĆÕæ©Ķ¬┐µĢ┤10µ»½ÕģŗńÜäµ║¢Õēć’╝īķĆɵ╝ĖĶ¬┐µĢ┤Ķć│µ»ÅÕż®õĖĆń▓Æ60µ»½ÕģŗńÜäÕł®ķĢĘĶāĮŃĆéńäČĶĆīõĖŹÕ╗║ĶŁ░Õ░ćÕŖæķćÅń╣╝ń║īÕó×ÕŖĀÕł░ĶČģķüÄ60µ»½Õģŗ/µ»ÅÕż®ŃĆé
[ 59]
ĶŚźµĢłõĮ£ńö©µÖéķ¢ō’╝Üń┤ä8Õ░ŵÖéŃĆé
’╝łPrevious Methylphenidate Dose’╝ē
’╝łRecommended Ritalin LA Dose’╝ē
µ»ÅÕż®µ£Źńö©5µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮõ║īµ¼Ī
µ»ÅÕż®õĖĆń▓Æ10µ»½ÕģŗńÜäÕł®ķĢĘĶāĮ ’╝ł10 mg q.d.’╝ē
µ»ÅÕż®µ£Źńö©10µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮõ║īµ¼Īµł¢20mgńÜämethylphenidate-SR
µ»ÅÕż®õĖĆń▓Æ20µ»½ÕģŗńÜäÕł®ķĢĘĶāĮ’╝ł20 mg q.d.’╝ē
µ»ÅÕż®µ£Źńö©15µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮõ║īµ¼Ī
µ»ÅÕż®õĖĆń▓Æ30µ»½ÕģŗńÜäÕł®ķĢĘĶāĮ ’╝ł30 mg q.d.’╝ē
µ»ÅÕż®µ£Źńö©20µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮõ║īµ¼Īµł¢40mgńÜämethylphenidate-SR
µ»ÅÕż®õĖĆń▓Æ40µ»½ÕģŗńÜäÕł®ķĢĘĶāĮ’╝ł40 mg q.d.’╝ē
µ»ÅÕż®µ£Źńö©30µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮõ║īµ¼Īµł¢60mgńÜämethylphenidate-SR
µ»ÅÕż®õĖĆń▓Æ60µ»½ÕģŗńÜäÕł®ķĢĘĶāĮ’╝ł60 mg q.d.’╝ē
[ 60]
Õ░łµĆØķüö µś»õĖĆń¦ŹÕōīńö▓ķģ»ń╝ōķćŖķĢ┐µĢłÕłČÕēé[ 61] µ│©µäÅń╝║ķÖĘÕżÜÕŖ©ķÜ£ńóŹ ’╝łADHD, Attention Deficit Hyperactivity Disorder’╝ēŃĆüÕÅæõĮ£µĆ¦ńØĪńŚģ ŃĆüÕÅīńøĖµāģµä¤ķÜ£ńóŹ ÕÆīķ揵ƦµŖæķāüķÜ£ńóŹ ŃĆé[ 3]
12µŁ▓õ╗źõĖŗńÜäõĮ┐ńö©ĶĆģ’╝īÕ░łµĆØķüöńÜ䵻ŵŚźµ£ĆÕż¦ÕŖæķćÅõĖŖķÖÉńé║54µ»½Õģŗ’╝ø13Õł░65µŁ▓ńÜäõĮ┐ńö©ĶĆģõ╣ŗµ»ÅµŚźµ£ĆÕż¦ÕŖæķćÅõĖŖķÖÉńé║72µ»½Õģŗ’╝īķØÆÕ░æÕ╣┤õĖŹĶČģĶ┐ćµ»ÅÕŹāÕģŗõĮōķćŹ2µ»½Õģŗ’╝ø[ 3]
Õ░łµĆØķüöÕł®ńö©µ╗▓ķĆÅÕŻō õ╗źµÄ¦ÕłČÕōīńö▓ķģ»ńÜäķćŗµöŠķƤÕ║”ŃĆéķĆÖķĀģń│╗ńĄ▒õ╗źÕé│ńĄ▒ķīĀÕŖæńé║Õż¢ÕĮó’╝īńö▒Õɽµ£ēÕ┐½ķƤķćŗµöŠÕ×ŗĶŚźńē®ńé║Õż¢ĶĪŻńÜäÕŹŖµ╗▓ķĆÅĶå£ÕīģĶ”åĶæŚµ╗▓ķĆÅµĆ¦ńÜäµ┤╗µĆ¦õĖēÕ▒żÕģ¦µĀĖµēĆńĄäµłÉŃĆé
µ┤╗µĆ¦õĖēÕ▒żÕģ¦µĀĖńÜäńĄäµłÉµś»Õģ®Õ▒żÕīģÕɽĶŚźńē®ÕÆīĶ│”ÕĮóÕŖæńÜäĶŚźńē®Õ▒żõ╗źÕÅŖõĖĆÕ▒żÕīģÕɽµ╗▓ķĆÅµĆ¦µ┤╗µĆ¦µłÉõ╗ĮńÜäµÄ©ĶŚźÕ▒żŃĆéÕ£©ķīĀÕŖæÕ░Šń½»ńÜäĶŚźńē®Õ▒żµ£ēõĖĆÕĆŗńö▒ń▓ŠÕ»åķøĘÕ░äķæĮÕŁöńÜäÕŁöµ┤×ŃĆéńĢČÕ£©µ£ēµ░┤ńÜäńÆ░ÕóāõĖŗ’╝īõŠŗÕ”éĶāāĶģĖķüōõĖŁ’╝īĶŚźńē®Õż¢ĶĪŻµ£āÕ£©õĖĆÕ░ŵÖéõ╣ŗÕģ¦µ║Čķøóõ╗źµÅÉõŠøĶĄĘÕ¦ŗÕŖæķćÅńÜäÕōīńö▓ķģ»ŃĆéµ░┤õ╗ĮńČōńö▒ķĆÖÕ▒żĶ壵╗▓ķĆÅĶć│ķīĀÕŖæńÜäÕģ¦µĀĖ’╝īÕ░ÄĶć┤µ╗▓ķĆÅµĆ¦µ┤╗µĆ¦ĶüÜÕÉłńē®õ╣ŗĶ│”ÕĮóÕŖæĶå©Ķä╣’╝īĶ«ōÕōīńö▓ķģ»ķĆÜķüÄĶ®▓ÕŁöµ┤×ķćŗµöŠÕć║ÕÄ╗ŃĆéķĆÖÕ▒żĶå£ĶŚēńö▒µ░┤ÕłåķĆ▓ÕģźķīĀÕŖæÕģ¦µĀĖńÜäµ¢╣Õ╝ÅµÄ¦ÕłČĶŚźńē®ķćŗÕć║ńÜäķƤÕ║”ŃĆ鵣żń│╗ńĄ▒ĶŚźńē®ķćŗÕć║ńÜäķƤÕ║”Õ£©µÄźõĖŗõŠåńÜä6Ķć│7Õ░ŵÖéµ£āķÜ©ĶæŚµÖéķ¢ōĶĆīÕó×ÕŖĀ’╝īĶĪƵ╝┐µ£Ćķ½śµ┐āÕ║”Õ╣│ÕØćÕ£©6Ķć│10Õ░ŵÖéõ╣ŗķ¢ō’╝īÕģČÕŠīµ£āķÜ©µÖéķ¢ōķĆɵ╝ĖõĖŗķÖŹ’╝īķīĀÕŖæõĖŁńÜäńö¤ńē®µā░µĆ¦µłÉÕłåÕ£©ķĆÜķüÄĶāāĶģĖķüōńÜäķüÄń©ŗõĖŁõ╗ŹńäČõ┐صīüÕ«īµĢ┤’╝īõĖöĶłćõĖŹÕÅ»µ║Čõ╣ŗĶŚźµĀĖµłÉõ╗ĮõĖĆĶĄĘõ╗źķīĀÕŖæĶŚźµ«╝ńÜäÕż¢ÕĮóķÜ©ĶæŚń│×õŠ┐µÄÆķÖżŃĆé[ 62]
ĶŚźµĢłµīüń║īµÖéķ¢ō’╝Üń┤äÕŹüõ║īÕ░ŵÖéŃĆé
[ 63]
µéŻĶĆģÕ╣┤ń┤Ć
Õ╗║ĶŁ░ĶĄĘÕ¦ŗÕŖæķćÅ
ÕŖæķćÅń»äÕ£Ź
6-12µŁ▓
18 µ»½Õģŗ/µ»ÅÕż®
18 - 54 µ»½Õģŗ/µ»ÅÕż®
13-17µŁ▓
18 µ»½Õģŗ/µ»ÅÕż®
18 - 72 µ»½Õģŗ/µ»ÅÕż® µ»ÅÕż®µ»ÅÕģ¼µ¢żõĖŹÕÅ»ĶČģķüÄ2µ»½ÕģŗŃĆé[ Ķ©╗ 1]
18-65µŁ▓
18 µ»½Õģŗ/µ»ÅÕż®
18 - 72 µ»½Õģŗ/µ»ÅÕż®
[ 51]
Õ£©Õ░łµĆØķüöńÜäĶŚźńē®Ķ®”ķ®ŚķüÄń©ŗõĖŁńÖ╝ńÅŠ’╝ī13-17µŁ▓ńÜäķØÆÕ╣┤Ķ®”ķ®ŚńĄäõĖŁ’╝īÕ░łµĆØķüöńÜäµ£ĆõĮĵ£ēµĢłÕŖæķćÅńé║’╝ܵ»ÅÕż®µ»ÅÕģ¼µ¢ż1.4µ»½ÕģŗŃĆé[ 64]
18µŁ▓õ╗źõĖŖńÜäÕģ®ÕĆŗµłÉõ║║Ķ®”ķ®ŚńĄäõĖŁ’╝īńÖ╝ńÅŠµ»ÅÕż®18-72µ»½ÕģŗńÜäÕŖæķćÅńÜåÕÅ»ķüöÕł░Õ£©ńĄ▒Ķ©łÕŁĖõĖŖÕģĘķĪ»ĶæŚµäÅńŠ®ńÜäńÖéµĢł’╝īńäČĶĆīõ╗źµ»ÅÕż®36µ»½Õģŗõ╗źõĖŖķĆ▓ĶĆīķüöÕł░ńĄ▒Ķ©łÕŁĖõĖŖÕģĘķĪ»ĶæŚµäÅńŠ®ńÜäńÖéµĢłńÜäĶć©Õ║ŖĶ®”ķ®ŚĶĆģńé║Õż¦ÕżÜµĢĖŃĆé [ 65]
ń¤ŁµĢłÕł®õ╗¢ĶāĮńÜäµ»ÅÕż®ńĖĮÕŖæķćÅ
Õ╗║ĶŁ░ĶĮēµÅøĶć│Õ░łµĆØķüöńÜäÕŖæķćÅ
µ»ÅÕż®µ£Źńö©5µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮõ║īĶć│õĖēµ¼Ī
µ»ÅÕż®µŚ®õĖŖµ£Źńö©õĖĆķĪå18µ»½ÕģŗńÜäÕ░łµĆØķüö
µ»ÅÕż®µ£Źńö©10µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮõ║īĶć│õĖēµ¼Ī
µ»ÅÕż®µŚ®õĖŖµ£Źńö©õĖĆķĪå36µ»½ÕģŗńÜäÕ░łµĆØķüö
µ»ÅÕż®µ£Źńö©15µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮõ║īĶć│õĖēµ¼Ī
µ»ÅÕż®µŚ®õĖŖµ£Źńö©õĖĆķĪå54µ»½ÕģŗńÜäÕ░łµĆØķüö
µ»ÅÕż®µ£Źńö©20µ»½ÕģŗńÜäÕł®õ╗¢ĶāĮõ║īĶć│õĖēµ¼Ī
µ»ÅÕż®µŚ®õĖŖµ£Źńö©õĖĆķĪå72µ»½ÕģŗńÜäÕ░łµĆØķüö
[ 51]
Ķ©╗Ķ¦Ż’╝Ü
ÕÅ░ńüŻÕ░ܵ£¬Õ╝ĢķĆ▓72µ»½Õģŗõ╣ŗÕ░łµĆØķüöŃĆé
Õ«ēõ┐ØńŠÄÕ¢£ķīĀ’╝łApo-Methylphenidate’╝ēńé║Õł®õ╗¢ĶāĮńÜäÕē»Õ╗ĀĶŚźÕōüŃĆé Õē»Õ╗ĀÕÉŹ’╝ÜApotex Incorporation. [ 52]
µéŻĶĆģµēĆķ£ĆńÜäĶŚźńē®µ▓╗ńÖéµÖéķ¢ōÕøĀõ║║ĶĆīńĢ░ŃĆéńäČĶĆīÕŁĖńĢīµÖ«ķüŹĶ¬Źńé║’╝īĶŚźńē®µ▓╗ńÖéķĆÜÕĖĖõĖŹĶāĮÕż¬ń¤ŁŃĆéµéŻĶĆģĶłćķå½ÕĖ½µćēĶ®▓իܵ£¤Ķ┐ĮĶ╣żĶć¬ÕĘ▒õ╗źÕÅŖµéŻĶĆģńÜäńŚģµ│üŃĆéµéŻĶĆģńÜäńŚćńŗƵ£ēÕÅ»ĶāĮÕ£©µÜ½µÖ鵳¢µ░Ėõ╣ģÕü£ĶŚźÕŠīń╣╝ń║īµö╣Õ¢ä’╝īÕĆśĶŗźÕ”鵣ż’╝īÕēćÕÅ»ĶāĮµś»Õü£ĶŚźµÖéµ®¤ķ╗×ŃĆé
[ 66]
Õ£©Õģ©ńÉāĶīāÕø┤Õåģ’╝īÕōīńö▓ķģ»µś»ŃĆŖń▓Šńź×ĶŹ»ńē®Õģ¼ń║” ŃĆŗõĖŁńÜäSchedule IIń▒╗ĶŹ»ńē®ŃĆé
Õ£©ńŠÄÕøĮ’╝īÕōīńö▓ķģ»Ķó½Õłåń▒╗õĖ║ķÖäĶĪ©II ÕÅŚń«ĪÕłČńē®Ķ┤©’╝īÕŹ│µ£ēÕģ¼Ķ«żńÜäÕī╗ń¢Śõ╗ĘÕĆ╝õĮåÕģʵ£ēķ½śńÜäµ╗źńö©ÕÅ»ĶāĮµĆ¦ŃĆé
Õ£©Ķŗ▒ÕøĮ’╝īÕōīńö▓ķģ»µś»Bń▒╗ÕÅŚń«ĪÕłČńē®Ķ┤©ŃĆ鵌ĀÕżäµ¢╣ĶĆīµīüµ£ēÕōīńö▓ķģ»ÕÅ»Õżäõ╗źµ£Ćķ½śõ║öÕ╣┤µ£ēµ£¤ÕŠÆÕłæÕÆī/µł¢µŚĀõĖŖķÖÉńÜäńĮܵ¼Š’╝īķØ×µ│ĢÕö«ÕŹ¢ÕÅ»Õżäõ╗źµ£Ćķ½ś14Õ╣┤µ£ēµ£¤ÕŠÆÕłæÕÆī/µł¢µŚĀõĖŖķÖÉńÜäńĮܵ¼ŠŃĆé
Õ£©ÕŖĀµŗ┐Õż¦’╝īÕōīńö▓ķģ»Ķó½ÕłŚÕģźŃĆŖÕÅŚń«ĪÕłČĶŚźńē®Ķłćńē®Ķ│¬µ│Ģõ╗ż ŃĆŗõĖŁńÜäń¼¼õĖēķĪ×ń«ĪÕłČĶŚźÕōü’╝łõĖÄLSDŃĆüĶ┐ĘÕ╣╗ĶśæĶÅćŃĆüķģȵ¢»ÕŹĪńüĄńŁēÕÉīõĖĆÕłåń▒╗’╝ē’╝īõĖöµŚĀÕżäµ¢╣µīüµ£ēÕōīńö▓ķģ»õĖ║ķØ×µ│ĢŃĆé
Õ£©µ¢░Ķź┐Õģ░’╝īÕōīńö▓ķģ»µś»B2ń▒╗ń«ĪÕłČńē®Ķ┤©’╝īķØ×µ│Ģµīüµ£ēÕÅ»Õżäõ╗źÕģŁõĖ¬µ£łµ£ēµ£¤ÕŠÆÕłæ’╝īķØ×µ│ĢÕć║Õö«ÕÅ»Õżäõ╗ź14Õ╣┤µ£ēµ£¤ÕŠÆÕłæŃĆé
Õ£©µŠ│Õż¦Õł®õ║Ü’╝īÕōīńö▓ķģ»µś»Schedule 8ń«ĪÕłČńē®Ķ┤©ŃĆ鵣żń▒╗ńē®Ķ┤©Õ£©Õć║Õö«ÕēŹÕ┐ģķĪ╗µöŠńĮ«Õ£©ÕÅ»õĖŖķöüńÜäõ┐ØķÖ®ń«▒Õåģ’╝īµŚĀÕżäµ¢╣µīüµ£ēÕÅ»Ķó½Õżäõ╗źÕĘ©ķóØńĮܵ¼ŠńöÜĶć│ńøæń”üŃĆé
Õ£©ńæ×ÕģĖ’╝īÕōīńö▓ķģ»µś»List IIń▒╗ń«ĪÕłČńē®Ķ┤©’╝īÕģʵ£ēÕģ¼Ķ«żńÜäÕī╗ń¢Śõ╗ĘÕĆ╝ŃĆ鵌ĀÕżäµ¢╣µīüµ£ēÕÅ»Ķó½Õżäõ╗źµ£Ćķ½śõĖēÕ╣┤ńÜäµ£ēµ£¤ÕŠÆÕłæŃĆé
Õ£©µ│ĢÕøĮ’╝īÕōīńö▓ķģ»Ķó½Õłåń▒╗õĖ║ķ║╗ķåēÕēé’╝īÕ╝ĆÕżäµ¢╣õĖÄÕö«ÕŹ¢Ķó½õĖźµĀ╝ń«ĪÕłČ’╝īÕŬĶāĮÕ£©Õ╝ĆÕć║Õżäµ¢╣ńÜäÕī╗ķÖóĶ┤Łõ╣░’╝łhospital-only prescription’╝ē’╝īńö©õ║ÄÕłØµ¼Īµ▓╗ń¢Śõ╗źÕÅŖµ»ÅÕ╣┤õĖĆÕ║”ńÜäÕÆ©Ķ»óŃĆé
Õ£©ÕŹ░Õ║”’╝īÕōīńö▓ķģ»µś»Schedule XĶŹ»ńē®õĖöÕÅŚ1945Õ╣┤ÕÅæÕĖāńÜäŃĆŖĶŹ»ńē®õĖÄÕī¢Õ”åÕōüµ│ĢµĪłŃĆŗń«ĪÕłČ’╝īÕŬĶāĮÕćŁńź×ń╗ÅńŚģõĖōÕ«Čµł¢ń▓Šńź×ń¦æÕī╗ńö¤Õ╝ĆÕć║ńÜäÕżäµ¢╣Ķ┤Łõ╣░ŃĆé
Õ£©ÕÅ░µ╣Š’╝īÕōīńö▓ķģ»õŠØõŠŗõĖ║ń¼¼õĖēń║¦ń«ĪÕłČĶŹ»Õōü’╝īÕī╗ÕĖłÕżäµ¢╣ķ£ĆõĮ┐ńö©ń«ĪÕłČĶŹ»ÕōüõĖōńö©Õżäµ¢╣ń¼║ŃĆé[ 67]
Õ£©õĖŁÕŹÄõ║║µ░æÕģ▒ÕÆīÕøĮ ’╝łÕż¦ķÖåÕ£░Õī║’╝ē’╝īĶ┐ÖõĖĆĶŹ»ńē®Õ▒×õ║Äń¼¼õĖĆń▒╗ń▓Šńź×ĶŹ»Õōü’╝ī[ 68] µ│©µäÅń╝║ķÖĘÕżÜÕŖ©ķÜ£ńóŹ ’╝īÕÅ»õĖƵ¼ĪÕżäµ¢╣30µŚźķćÅ’╝łĶĆīķØ×õĖĆĶł¼ń▓Šķ║╗ĶŹ»ÕōüµÄ¦ń╝ōķćŖÕłČÕēéńÜä7µŚźķćÅ’╝ēŃĆé[ 69] [ 70]
^ Kimko, HC; Cross, JT; Abernethy, DR. Pharmacokinetics and clinical effectiveness of methylphenidate.. Clinical pharmacokinetics. December 1999, 37 (6): 457ŌĆō70. PMID 10628897 doi:10.2165/00003088-199937060-00002 ^ 2.0 2.1 Methylphenidate. Pubchem Compound. National Center for Biotechnology Information. ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2014-01-06’╝ē . ^ 3.0 3.1 3.2 FDA. CONCERTA(methylphenidate HCl) Extended-release Tablets CII PDF’╝ł167 KB’╝ē 2007
^ Label of Ritalin . DailyMed. Novartis. 2017-01-05 [March, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-20’╝ē. Methylphenidate hydrochloride USP is a white, odorless, fine crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77. ^ www.ehow.com/about_5374709_ritalin-invented.html When Was Ritalin Invented?, citing Lawrence Diller: "Running on Ritalin", 1999
^
Diller, Lawrence. Running on Ritalin. 1999. ISBN 978-0553379068
^
Lange KW, Reichl S, Lange KM, Tucha L, Tucha O. The history of attention deficit hyperactivity disorder . ADHD Attention Deficit and Hyperactivity Disorders. 2010, 2 (4): 241ŌĆō55. PMC 3000907 PMID 21258430 doi:10.1007/s12402-010-0045-8
^ ADHD drug use 'up 50% in six years' . 2013-08-13 [2017-03-29 ] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-09-21’╝ē –ķĆÜĶ┐ćwww.bbc.com. ^ Narcotics monitoring board reports 66% increase in global consumption of methylphenidate . [2017-03-29 ] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-09-21’╝ē. ^ "Ritalin & Cocaine: The Connection and the Controversy" ’╝łķĪĄķØóÕŁśµĪŻÕżćõ╗Į ’╝īÕŁśõ║Äõ║ÆĶüöńĮæµĪŻµĪłķ”å ’╝ē. Learn.genetics.utah.edu. Retrieved on 2011-10-16.^ Mary Ann Boyd. Psychiatric nursing: contemporary practice . Lippincott Williams & Wilkins. 2005: 160ŌĆō [2011-04-30 ] . ISBN 978-0-7817-4916-9ÕŁśµĪŻ õ║Ä2015-04-14’╝ē. ^ Peter Doskoch. Why isn't methylphenidate more addictive? . NeuroPsychiatry Rev. 2002, 3 (1): 19 [2013-01-24 ] . ’╝łÕĤզŗÕåģÕ«╣ ÕŁśµĪŻõ║Ä2009-03-30’╝ē. ^ Functional Roles of Norepinephrine and Dopamine in ADHD: Dopamine in ADHD . Medscape. 2006 [2013-10-08 ] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-09-08’╝ē. Catecholamines not only facilitate attention, they are essential to executive function. The prefrontal cortex directs behaviors, thoughts, and feelings represented in working memory. This representational knowledge is essential to fundamental cognitive abilities that compromise executive functions. These encompass the ability to (1) inhibit inappropriate behaviors and thoughts, (2) regulate our attention, (3) monitor our actions, and (4) plan and organize for the future. Difficulties with these prefrontal cortex functions are evident in neuropsychological and imaging studies of ADHD patients and account for many of the common behavioral symptoms. Measures of prefrontal cortical functioning in animals indicate that these functions are sensitive to small changes in catecholamine modulation of prefrontal cortex cells that can produce profound effects on the ability of the prefrontal cortex to guide behavior. Optimal levels of NE acting at postsynaptic alpha2A-adrenoceptors and dopamine acting at D1 receptors are essential to prefrontal cortex function. Blockade of norepinephrine alpha2-adrenoceptors in prefrontal cortex markedly impairs prefrontal cortex function and mimics most of the symptoms of ADHD, including impulsivity and locomotor hyperactivity. Conversely, stimulation of prefrontal cortical alpha2-adrenoceptors strengthens prefrontal cortex regulation of behavior and reduces distractibility. Thus, effective treatments for ADHD facilitate catecholamine transmission and apparently have their therapeutic actions by optimizing catecholamine actions in the prefrontal cortex ^
Arnsten AF, Li BM. Neurobiology of Executive Functions: Catecholamine Influences on Prefrontal Cortical Functions. Biological Psychiatry. 2005, 57 (11): 1377ŌĆō84. PMID 15950011 doi:10.1016/j.biopsych.2004.08.019
^
Markowitz JS, DeVane CL, Ramamoorthy S, Zhu HJ. The psychostimulant d-threo-(R,R)-methylphenidate binds as an agonist to the 5HT(1A) receptor.. Pharmazie. Feb 2009, 64 : 123ŌĆō5. PMID 19322953
^ Fry JM. Treatment modalities for narcolepsy. Neurology. February 1998, 50 (2 Suppl 1): S43ŌĆōS48. PMID 9484423 S2CID 36824088 doi:10.1212/WNL.50.2_Suppl_1.S43 ^ Mitler MM. Evaluation of treatment with stimulants in narcolepsy. Sleep. December 1994, 17 (8 Suppl): S103ŌĆōS106. PMID 7701190 doi:10.1093/sleep/17.suppl_8.S103 ^ Markowitz, JS; Patrick, KS. Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers: does chirality matter?. Journal of Clinical Psychopharmacology. June 2008, 28 (3 Suppl 2): S54ŌĆō61. PMID 18480678 doi:10.1097/JCP.0b013e3181733560 ^ Williard, RL; Middaugh, LD; Zhu, HJ; Patrick, KS. Methylphenidate and its ethanol transesterification metabolite ethylphenidate: brain disposition, monoamine transporters and motor activity.. Behavioural Pharmacology. February 2007, 18 (1): 39ŌĆō51. PMID 17218796 doi:10.1097/fbp.0b013e3280143226 ^ 20.0 20.1 Markowitz, JS; DeVane, CL; Pestreich, LK; Patrick, KS; Muniz, R. A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study.. Journal of child and adolescent psychopharmacology. December 2006, 16 (6): 687ŌĆō98. PMID 17201613 doi:10.1089/cap.2006.16.687 Õ╝Ģńö©ķöÖĶ»»’╝ÜÕĖ”µ£ēnameÕ▒׵ƦŌĆ£pmid17201613ŌĆØńÜä<ref>µĀćńŁŠńö©õĖŹÕÉīÕåģÕ«╣Õ«Üõ╣ēõ║åÕżÜµ¼Ī ^ 21.0 21.1 Heal DJ, Pierce DM. Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system. CNS Drugs. 2006, 20 (9): 713ŌĆō38. PMID 16953648 doi:10.2165/00023210-200620090-00002 ^ Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology . British Journal of Pharmacology. 2006, 147 (Suppl 1): S82ŌĆō8. PMC 1760736 PMID 16402124 doi:10.1038/sj.bjp.0706428 ^ Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review (PDF) . Prog. Neurobiol. 2005, 75 (6): 406ŌĆō33 [2013-01-21 ] . PMID 15955613 doi:10.1016/j.pneurobio.2005.04.003 ÕĤզŗÕåģÕ«╣ (PDF) ÕŁśµĪŻõ║Ä2020-10-24’╝ē. ^ Viggiano D, Vallone D, Sadile A. Dysfunctions in dopamine systems and ADHD: evidence from animals and modeling . Neural Plasticity. 2004, 11 (1ŌĆō2): 102, 106ŌĆō107. PMC 2565441 PMID 15303308 doi:10.1155/NP.2004.97 [1] ’╝łķĪĄķØóÕŁśµĪŻÕżćõ╗Į ’╝īÕŁśõ║Äõ║ÆĶüöńĮæµĪŻµĪłķ”å ’╝ē^ Novartis: Focalin XR Overview ’╝łķĪĄķØóÕŁśµĪŻÕżćõ╗Į ’╝īÕŁśõ║Äõ║ÆĶüöńĮæµĪŻµĪłķ”å ’╝ē
^ Focalin XR ŌĆō Full Prescribing Information ’╝łķĪĄķØóÕŁśµĪŻÕżćõ╗Į ’╝īÕŁśõ║Äõ║ÆĶüöńĮæµĪŻµĪłķ”å ’╝ē. Novartis.^ SPC Concerta XL 18 mg ŌĆō 36 mg prolonged release tablets ’╝łķĪĄķØóÕŁśµĪŻÕżćõ╗Į ’╝īÕŁśõ║Äõ║ÆĶüöńĮæµĪŻµĪłķ”å ’╝ē last updated on the eMC: 05/11/2010^ T. J. Volz. Neuropharmacological Mechanisms Underlying the Neuroprotective Effects of Methylphenidate . Current Neuropharmacology. 2008 [2013-01-21 ] . PMC 2701286 doi:10.2174/157015908787386041 ÕŁśµĪŻ õ║Ä2019-04-12’╝ē. ^ Gordon N. Attention deficit hyperactivity disorder: possible causes and treatment. Int. J. Clin. Pract. 1999, 53 (7): 524ŌĆō8. PMID 10692738 ^ King S, Griffin S, Hodges Z; et al. A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents . Health Technol Assess. 2006, 10 (23): iiiŌĆōiv, xiiiŌĆō146 [2013-01-21 ] . PMID 16796929 ÕĤզŗÕåģÕ«╣ ÕŁśµĪŻõ║Ä2012-08-02’╝ē. ^ Gonzalez de Dios J, Card├│ E, Servera M. [Methylphenidate in the treatment of attention-deficit/hyperactivity disorder: are we achieving an adequate clinical practice?]. Rev Neurol. 2006, 43 (12): 705ŌĆō14. PMID 17160919 ’╝łĶź┐ńÅŁńēÖĶ»Ł’╝ē . ^ ŌĆō Ritalin Side Effects ’╝łķĪĄķØóÕŁśµĪŻÕżćõ╗Į ’╝īÕŁśõ║Äõ║ÆĶüöńĮæµĪŻµĪłķ”å ’╝ē. Drugs.com. Retrieved on 2011-10-16.^ Jaanus SD. Ocular side-effects of selected systemic drugs. Optom Clin. 1992, 2 (4): 73ŌĆō96. PMID 1363080 ^ Auger RR, Goodman SH, Silber MH, Krahn LE, Pankratz VS, Slocumb NL. Risks of high-dose stimulants in the treatment of disorders of excessive somnolence: a case-control study. Sleep. 2005, 28 (6): 667ŌĆō72. PMID 16477952 ^ Concerta product monograph (PDF) . Janssen Pharmaceuticals. [2016-12-04 ] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ (PDF) õ║Ä2017-01-28’╝ē. ^ Patrick KS, Gonz├Īlez MA, Straughn AB, Markowitz JS. New methylphenidate formulations for the treatment of attention-deficit/hyperactivity disorder. Expert Opinion on Drug Delivery. 2005, 2 (1): 121ŌĆō43. PMID 16296740 doi:10.1517/17425247.2.1.121 ^ 37.0 37.1
Markowitz JS, DeVane CL, Boulton DW, Nahas Z, Risch SC, Diamond F, Patrick KS. Ethylphenidate formation in human subjects after the administration of a single dose of methylphenidate and ethanol. Drug metabolism and disposition: the biological fate of chemicals. 2000, 28 (6): 620ŌĆō4. PMID 10820132
^
Markowitz JS, Logan BK, Diamond F, Patrick KS. Detection of the novel metabolite ethylphenidate after methylphenidate overdose with alcohol coingestion. Journal of Clinical Psychopharmacology. 1999, 19 (4): 362ŌĆō6. PMID 10440465 doi:10.1097/00004714-199908000-00013
^
Patrick KS, Straughn AB, Minhinnett RR, Yeatts SD, Herrin AE, DeVane CL, Malcolm R, Janis GC, Markowitz JS. Influence of ethanol and gender on methylphenidate pharmacokinetics and pharmacodynamics. . Clinical pharmacology and therapeutics. March 2007, 81 (3): 346ŌĆō53. PMC 3188424 PMID 17339864 doi:10.1038/sj.clpt.6100082
^
Roberts SM, DeMott RP, James RC. Adrenergic modulation of hepatotoxicity. Drug Metab. Rev. 1997, 29 (1ŌĆō2): 329ŌĆō53. PMID 9187524 doi:10.3109/03602539709037587
^ Chi-Yung Shang, Yi-Lei Pan, Hsiang-Yuan Lin, Lin-Wan Huang & Susan Shur-Fen Gau. An Open-Label, Randomized Trial of Methylphenidate and Atomoxetine Treatment in Children with Attention-Deficit/Hyperactivity Disorder. Journal of child and adolescent psychopharmacology. September 2015, 25 (7): 566ŌĆō573. PMID 26222447 doi:10.1089/cap.2015.0035 At week 24, mean changes in ADHD-RS-IV Inattention scores were 13.58 points (Cohen's d, -3.08) for OROS-methylphenidate and 12.65 points (Cohen's d, -3.05) for atomoxetine; and mean changes in ADHD-RS-IV Hyperactivity-Impulsivity scores were 10.16 points (Cohen's d, -1.75) for OROS-methylphenidate and 10.68 points (Cohen's d, -1.87) for atomoxetine. ^ õĖŁĶÅ»µ░æÕ£ŗĶĪøńö¤ń”ÅÕł®ķā©/Õ┐āńÉåĶĪøńö¤Õ░łĶ╝»/03µ│©µäÅÕŖøõĖŹĶČ│ķüÄÕŗĢńŚć.pdf (PDF) . http://www.mohw.gov.tw/ . õĖŁĶÅ»µ░æÕ£ŗĶĪøńö¤ń”ÅÕł®ķā©. June 2015 [February 2017] . ’╝łÕĤզŗÕåģÕ«╣ (PDF) ÕŁśµĪŻõ║Ä2017-02-19’╝ē. õĖĆĶł¼ńÖ╝ńÅŠÕģČÕ░Źµ¢╝Õ░łµ│©Õ║”ńÜäµö╣Õ¢äµ▓Ƶ£ē MPH µśÄķĪ» ^ 3, µ│©µäÅÕŖøõĖŹĶČ│ķüÄÕŗĢńŚć (PDF) , õĖŁĶÅ»µ░æÕ£ŗĶĪøńö¤ń”ÅÕł®ķā©/Õ┐āńÉåĶĪøńö¤Õ░łĶ╝» 1 1, õĖŁĶÅ»µ░æÕ£ŗĶĪøńö¤ń”ÅÕł®ķā©/Õ┐āńÉåĶĪøńö¤Õ░łĶ╝»/03µ│©µäÅÕŖøõĖŹĶČ│ķüÄÕŗĢńŚć.pdf: õĖŁĶÅ»µ░æÕ£ŗĶĪøńö¤ń”ÅÕł®ķā©: 22, [June 2015] [2020-10-04 ] , ISBN 9789860454154ÕĤզŗÕåģÕ«╣ (PDF) ÕŁśµĪŻõ║Ä2017-02-19’╝ē ’╝łõĖŁµ¢ć’╝łń╣üķ½ö’╝ē’╝ē , atomoxetine’╝īńö©Õ£©ńŚģµāģ Ķ╝āńé║Ķżćķø£ŃĆüµł¢µś»ńäĪµ│ĢÕ┐ŹÕÅŚMPHÕē»õĮ£ńö©ńÜäµéŻĶĆģ’╝īńäČĶĆīõĖĆĶł¼ńÖ╝ńÅŠÕģČÕ░Źµ¢╝Õ░łµ│©Õ║”ńÜäµö╣Õ¢äµ▓Ƶ£ēMPHµśÄķĪ» ^ Parent's Medication Guide: ADHD . American Psychiatric Association (Guidelines (Tertiary source)). American Psychiatric Association & American Academy of Child and Adolescent Psychiatry (AACAP). July 2013 [January 2017] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-02-02’╝ē. Though not FDA-approved for combined treatment, atomoxetine (Strattera) is sometimes used in conjunction with stimulants as an off-label combination therapy. ^ Medical Encyclopedia ŌåÆ Attention deficit hyperactivity disorder . MedlinePlus.gov. 2017-01-05 [January 2017] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-01-26’╝ē. Medicine combined with behavioral treatment often works best. Different ADHD medicines can be used alone or combined with each other. The doctor will decide which medicine is right, based on the person's symptoms and needs. ^ Treuer T, Gau SS, M├®ndez L, Montgomery W, Monk JA, Altin M; et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. . Journal of Child and Adolescent Psychopharmacology (systematic review (Secondary source)). 2013, 23 (3): 179ŌĆō93. PMC 3696926 PMID 23560600 doi:10.1089/cap.2012.0093 Existing evidence suggests, but does not confirm, that this drug combination may benefit some, but not all, patients who have tried several ADHD medications without success. ^ Perugi G, Vannucchi G. The use of stimulants and atomoxetine in adults with comorbid ADHD and bipolar disorder. . Expert Opin Pharmacother. 2015, 16 (14): 2193ŌĆō204. PMID 26364896 doi:10.1517/14656566.2015.1079620 Although systematic trials on the use of stimulants and ATX in ADHD-BD comorbidity in adulthood are necessary, both treatments should be considered possible options to be carefully evaluated once the patient has been stabilized. ^ Label of Strattera consisting of atomoxetine . DailyMed.gov (Leaflet/label (Tertiary source)). Eli Lilly Company. June 2015 [February 2017] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2018-06-07’╝ē. 7.7 Methylphenidate\ Coadministration of methylphenidate with STRATTERA did not increase cardiovascular effects beyond those seen with methylphenidate alone. ^ Label of Ritalin . DailyMed. Novartis. 2017-01-05 [March, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-20’╝ē. ^ Label of Ritalin LA (PDF) . Novartis.com. Novartis. Mid 2015 [January, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ (PDF) õ║Ä2017-08-30’╝ē. ^ 51.0 51.1 51.2 Label of Concerta (PDF) . concerta.net. Jassen Cilag. 2013 [January, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ (PDF) ÕŁśµĪŻõ║Ä2017-01-17’╝ē. ^ 52.0 52.1 Apotex Incorporation., Õ«ēõ┐ØńŠÄÕ¢£ķīĀ 10 µ»½Õģŗ ĶĪøńĮ▓ĶŚźĶ╝ĖÕŁŚń¼¼ 025016 ĶÖ¤ , ķ┤╗µ▒Čķå½ĶŚźÕ»”µźŁµ£ēķÖÉÕģ¼ÕÅĖ (ń╝¢), Information for the patient (PDF) , Canada, 2006-03-27 [2017-03-19 ] , ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ (PDF) õ║Ä2009-11-22’╝ē ^ Õ«ēõ┐ØńŠÄÕ¢£ķīĀ 10 µ»½Õģŗ ĶĪøńĮ▓ĶŚźĶ╝ĖÕŁŚń¼¼ 025016 ĶÖ¤ (PDF) , Canada: Apotex Incorporation., 2006-03-27 [2017-03-19 ] , ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ (PDF) õ║Ä2009-11-22’╝ē ^ Label of Ritalin LA . DailyMed.com. Novartis. Mid 2015 [January, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-26’╝ē. Dosing Recommendations’╝ÜDosage should be individualized according to the needs and responses of the patients.’╝ē ^ Label of Ritalin . DailyMed. Novartis. 2017-01-05 [March, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-20’╝ē. Ritalin hydrochloride, methylphenidate hydrochloride USP, is a mild central nervous system (CNS) stimulant, available as tablets of 5, 10, and 20 mg for oral administration; ^ Label of Ritalin . DailyMed.com. Novartis. 2017-01-05 [March, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-20’╝ē. Dosage should be individualized according to the needs and responses of the patient.
Adults
Tablets: Administer in divided doses 2 or 3 times daily, preferably 30 to 45 minutes before meals. Average dosage is 20 to 30 mg daily. Some patients may require 40 to 60 mg daily. In others, 10 to 15 mg daily will be adequate. Patients who are unable to sleep if medication is taken late in the day should take the last dose before 6 p.m.
SR Tablets: Ritalin-SR tablets have a duration of action of approximately 8 hours. Therefore, Ritalin-SR tablets may be used in place of Ritalin tablets when the 8-hour dosage of Ritalin-SR corresponds to the titrated 8-hour dosage of Ritalin. Ritalin-SR tablets must be swallowed whole and never crushed or chewed. ^ Label of Ritalin . DailyMed.com. Novartis. 2017-01-05 [March, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-20’╝ē. Dosage should be individualized according to the needs and responses of the patient.
Children (6 years and over)
Ritalin should be initiated in small doses, with gradual weekly increments. Daily dosage above 60 mg is not recommended.
If improvement is not observed after appropriate dosage adjustment over a 1-month period, the drug should be discontinued.
Tablets: Start with 5 mg twice daily (before breakfast and lunch) with gradual increments of 5 to 10 mg weekly.
SR Tablets: Ritalin-SR tablets have a duration of action of approximately 8 hours. Therefore, Ritalin-SR tablets may be used in place of Ritalin tablets when the 8-hour dosage of Ritalin-SR corresponds to the titrated 8-hour dosage of Ritalin. Ritalin-SR tablets must be swallowed whole and never crushed or chewed. ^ Label of Ritalin LA . DailyMed.com. Novartis. Mid 2015 [January, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-26’╝ē. Methylphenidate hydrochloride is a central nervous system (CNS) stimulant.
Ritalin LA┬« (methylphenidate hydrochloride) extended-release capsules is an extended-release formulation of methylphenidate with a bi-modal release profile. Ritalin LA uses the proprietary SODAS┬« (Spheroidal Oral Drug Absorption System) technology. Each bead-filled Ritalin LA capsule contains half the dose as immediate-release beads and half as enteric-coated, delayed-release beads, thus providing an immediate release of methylphenidate and a second delayed release of methylphenidate. Ritalin LA 10, 20, 30, 40, and 60 mg capsules provide in a single dose the same amount of methylphenidate as dosages of 5, 10, 15, 20, or 30 mg of Ritalin tablets given b.i.d.’╝ē ^ Label of Ritalin LA . DailyMed.com. Novartis. Mid 2015 [January, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-26’╝ē. Initial Treatment
The recommended starting dose of Ritalin LA is 20 mg once daily. Dosage may be adjusted in weekly 10 mg increments to a maximum of 60 mg/day taken once daily in the morning, depending on tolerability and degree of efficacy observed. Daily dosage above 60 mg is not recommended. When in the judgement of the clinician a lower initial dose is appropriate, patients may begin treatment with Ritalin LA 10 mg.’╝ē ^ Label of Ritalin LA . DailyMed.com. Novartis. Mid 2015 [January, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-26’╝ē. Initial Treatment
The recommended starting dose of Ritalin LA is 20 mg once daily. Dosage may be adjusted in weekly 10 mg increments to a maximum of 60 mg/day taken once daily in the morning, depending on tolerability and degree of efficacy observed. Daily dosage above 60 mg is not recommended. When in the judgement of the clinician a lower initial dose is appropriate, patients may begin treatment with Ritalin LA 10 mg.’╝ē ^ Ķīāµ┤▓ķÖģŃĆüµØÄÕģüµŁ”. µ│©µäÅń╝║ķÖĘÕżÜÕŖ©ķÜ£ńóŹńÜäµ▓╗ń¢ŚĶŹ»ńē®Ķ┐øÕ▒Ģ PDF’╝ł242 KB’╝ē . õĖŖµĄĘõ║żķĆÜÕż¦ÕŁ”Õī╗ÕŁ”ķÖóķÖäÕ▒×ń¼¼õ╣Øõ║║µ░æÕī╗ķÖó. 2007.
^ Concerta (Methylphenidate Extended-Release Tablets): Uses, Dosage, Side Effects, Interactions, Warning . RxList. [2020-04-20 ] . ’╝łÕĤզŗÕåģÕ«╣ ÕŁśµĪŻõ║Ä2021-11-19’╝ē ’╝łĶŗ▒Ķ»Ł’╝ē . ^ Label of Concerta . DailyMed.gov. Jassen Cilag. 2013 [January, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-26’╝ē. 1 INDICATIONS AND USAGE \
CONCERTA® is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in children 6 years of age and older, adolescents, and adults up to the age of 65 [see CLINICAL STUDIES (14)].
A diagnosis of Attention Deficit Hyperactivity Disorder (ADHD; DSM-IV) implies the presence of hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and be present in two or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive Type, at least six of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least six of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; "on the go;" excessive talking; blurting answers; can't wait turn; intrusive. The Combined Type requires both inattentive and hyperactive-impulsive criteria to be met. ^ Label of Concerta . DailyMed.gov. Jassen Cilag. 2013 [January, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-26’╝ē. 14.2 Adolescents
In a randomized, double-blind, multicenter, placebo-controlled trial (Study 4) involving 177 patients, CONCERTA┬« was demonstrated to be effective in the treatment of ADHD in adolescents aged 13 to 18 years at doses up to 72 mg/day (1.4 mg/kg/day). Of 220 patients who entered an open 4-week titration phase, 177 were titrated to an individualized dose (maximum of 72 mg/day) based on meeting specific improvement criteria on the ADHD Rating Scale and the Global Assessment of Effectiveness with acceptable tolerability. Patients who met these criteria were then randomized to receive either their individualized dose of CONCERTA┬« (18 ŌĆō 72 mg/day, n=87) or placebo (n=90) during a two-week double-blind phase. At the end of this phase, mean scores for the investigator rating on the ADHD Rating Scale demonstrated that CONCERTA┬« was statistically significantly superior to placebo. ^ Label of Concerta . DailyMed.gov. Jassen Cilag. 2013 [January, 2017.] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-26’╝ē. 14.2 Adolescents
14.3 Adults
Two double-blind, placebo-controlled studies were conducted in 627 adults aged 18 to 65 years. The controlled studies compared CONCERTA® administered once daily and placebo in a multicenter, parallel-group, 7-week dose-titration study (Study 5) (36 to 108 mg/day) and in a multicenter, parallel-group, 5-week, fixed-dose study (Study 6) (18, 36, and 72 mg/day).
Study 5 demonstrated the effectiveness of CONCERTA® in the treatment of ADHD in adults aged 18 to 65 years at doses from 36 mg/day to 108 mg/day based on the change from baseline to final study visit on the Adult ADHD Investigator Rating Scale (AISRS). Of 226 patients who entered the 7-week trial, 110 were randomized to CONCERTA® and 116 were randomized to placebo. Treatment was initiated at 36 mg/day and patients continued with incremental increases of 18 mg/day (36 to 108 mg/day) based on meeting specific improvement criteria with acceptable tolerability. At the final study visit, mean change scores (LS Mean, SEM) for the investigator rating on the AISRS demonstrated that CONCERTA® was statistically significantly superior to placebo.
Study 6 was a multicenter, double-blind, randomized, placebo-controlled, parallel-group, dose-response study (5-week duration) with 3 fixed-dose groups (18, 36, and 72 mg). Patients were randomized to receive CONCERTA┬« administered at doses of 18 mg (n=101), 36 mg (n=102), 72 mg/day (n=102), or placebo (n=96). All three doses of CONCERTA┬« were statistically significantly more effective than placebo in improving CAARS (Conners' Adult ADHD Rating Scale) total scores at double-blind end point in adult subjects with ADHD. ^ Label of Ritalin LA . DailyMed.com. Novartis. 2015 [January 2017] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2017-03-26’╝ē. Maintenance/Extended Treatment\There is no body of evidence available from controlled trials to indicate how long the patient with ADHD should be treated with Ritalin LA. It is generally agreed, however, that pharmacological treatment of ADHD may be needed for extended periods. Nevertheless, the physician who elects to use Ritalin LA for extended periods in patients with ADHD should periodically re-evaluate the long-term usefulness of the drug for the individual patient with trials off medication to assess the patientŌĆÖs functioning without pharmacotherapy. Improvement may be sustained when the drug is either temporarily or permanently discontinued.’╝ē ^ ń«ĪÕłČĶŚźÕōüÕłåń┤ÜÕÅŖÕōüķĀģ . [2017-01-02 ] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2016-05-13’╝ē. ^ ÕøĮÕ«ČķŻ¤ÕōüĶŹ»ÕōüńøæńØŻń«ĪńÉåÕ▒Ć. ń▓Šńź×ĶŹ»ÕōüÕōüń¦Źńø«ÕĮĢ’╝ł2007Õ╣┤ńēł’╝ē . Õī╗ĶŹ»ńĮæ. 2007-10-31 [2013-02-04 ] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2020-07-02’╝ē. ^ Õģ│õ║ÄÕ╗ČķĢ┐µ┤Šķåŗńö▓ķģ»ń╝ōķćŖÕēéµ▓╗ń¢Śµ│©µäÅń╝║ķÖĘÕżÜÕŖ©ķÜ£ńóŹÕżäµ¢╣ķÖÉիܵŚČķŚ┤ńÜäķĆÜń¤ź_ĶŹ»õ║ŗµö┐ńŁ¢ÕÅŖµ│ĢĶ¦ä_ĶĄäĶ«»õĖŁÕ┐ā_µ╣¢ÕŹŚĶŹ»õ║ŗµ£ŹÕŖĪńĮæ . www.hnysfww.com. [2021-07-08 ] . ’╝łÕĤզŗÕåģÕ«╣ÕŁśµĪŻ õ║Ä2021-08-03’╝ē. ^ ĶĮ¼ÕÅæń£üÕŹ½ńö¤ÕÄģÕŖ×Õģ¼Õ«żĶĮ¼ÕÅæÕŹ½ńö¤ķā©ÕŖ×Õģ¼ÕÄģÕģ│õ║ÄÕ╗ČķĢ┐µ┤Šķåŗńö▓ķģ»ń╝ōķćŖÕēéµ▓╗ń¢Śµ│©µäÅń╝║ķÖĘÕżÜÕŖ©ķÜ£ńóŹÕżäµ¢╣ķÖÉիܵŚČķŚ┤ńÜäķĆÜń¤ź . µ░ĖõĖ░ÕÄ┐ÕŹ½ńö¤Õ▒ĆÕŖ×Õģ¼Õ«ż. 2012-02-09. ’╝łÕĤզŗÕåģÕ«╣ ÕŁśµĪŻõ║Ä2021-10-24’╝ē.
µ┐ĆÕŗĢÕŖæ µŗ«µŖŚÕŖæ µ£¬ń¤źńē®/